10-K: Annual report pursuant to Section 13 and 15(d)
Published on August 28, 2007
Table of Contents
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 10-K
[X] ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(D) OF
THE SECURITIES EXCHANGE ACT OF 1934
For the fiscal year ended June 30, 2007
Commission file number: 001-15317
RESMED INC.
(Exact name of registrant as specified in its Charter)
Delaware
(State or other jurisdiction of incorporation or organization)
98-0152841
(IRS Employer Identification No.)
14040 Danielson Street
Poway, CA 92064-6857
United States of America
(Address of principal executive offices)
(858) 746-2400
(Registrants telephone number, including area code)
Securities registered pursuant to Section 12(b) of the Act
Title of each class
Common Stock, $.004 Par Value
Name of each exchange upon which registered
New York Stock Exchange
Securities registered pursuant to Section 12(g) of the Act
None
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes [ x ] No [ ]
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or 15(d) of the Act. Yes [ ] No [ x ]
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes [ x ] No [ ]
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulations S-K (S 229.405 of this Chapter) is not contained herein and will not be contained to the best of registrants knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K [ ]
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, or a non-accelerated filer. See definition of accelerated filer and large accelerated filer in Rule 12b-2 of the Exchange Act.
| Large accelerated filer [ x ] | Accelerated filer [ ] | Non-accelerated filer [ ] |
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). Yes [ ] No [ x ]
The aggregate market value of the voting stock held by non-affiliates of registrant as of December 31, 2006 (the last business day of the registrants most recently completed second fiscal quarter), computed by reference to the closing sale price of such stock on the New York Stock Exchange, was approximately $3,684,206,000. (All directors, executive officers, and 10% stockholders of registrant are considered affiliates.)
At August 17, 2007, registrant had 77,485,037 shares of Common Stock, $.004 par value, issued and outstanding. This number excludes 2,657,518 shares held by the registrant as treasury shares.
Portions of the registrants definitive Proxy Statement to be delivered to shareholders in connection with the registrants 2007 Annual Meeting of Stockholders, to be filed subsequent to the date hereof, are incorporated by reference into Part III of this report.
Table of Contents
Activa, ActiveCell, Adapt SV, Adaptiv, Aerial, Aero-Click, Aero-Fix, ApneaLink, AutoVPAP, AutoScan, AutoSet, AutoSet CS, AutoSet Spirit, AutoSet T, AutoSet Vantage, AutoSet.com, AutoSet-CS.com, AutoView, Bubble Cushion, Bubble Mask, Elisée, Eole, Escape, Helia, HumidAire, IPAP MAX, IPAP MIN, Kidsta, Magellan, Malibu, MAP, MEPAL, Meridian, MESAM, minni Max, Mirage, Protégé, Moritz biLEVEL, Papillon, Poly-MESAM, ResCap, ResControl, ResMed, S6, S7, S8, SELFSET, SleepVantage, SmartStart, Spirit, Spiro+, Sullivan, Swift, Tango, TiControl, Ultra Mirage, Vential, VPAP, VS Easyfit are our trademarks.
As used in this 10-K, the terms we, us, our and the Company refer to ResMed Inc., a Delaware corporation, and its subsidiaries, on a consolidated basis, unless otherwise stated.
- 1 -
Table of Contents
PART I
Cautionary Note Regarding Forward-Looking Statements
This report contains or may contain certain forward-looking statements and information that are based on the beliefs of our management as well as estimates and assumptions made by, and information currently available to our management. All statements other than statements regarding historical facts are forward-looking statements. The words believe, expect, anticipate, intend, seek, will, will continue, estimate, plan, future and other similar expressions generally identify forward-looking statements, including, in particular, statements regarding the development and approval of new products and product applications, market expansion, pending litigation, and the development of new markets for our products, such as cardiovascular and stroke markets. These forward-looking statements are made pursuant to the safe harbor provisions of the Private Securities Litigation Reform Act of 1995. You are cautioned not to place undue reliance on these forward-looking statements each of which applies only as of the date of this report. Such forward-looking statements reflect the views of our management at the time such statements are made and are subject to a number of risks, uncertainties, estimates and assumptions, including, without limitation, and in addition to those identified in the text surrounding such statements, those identified in Item 1A Risk Factors and elsewhere in this report.
In addition, important factors to consider in evaluating such forward-looking statements include changes or developments in social, economic, market, legal or regulatory circumstances, changes in our business or growth strategy or an inability to execute our strategy due to changes in our industry or the economy generally, the emergence of new or growing competitors, the actions or omissions of third parties, including suppliers, customers, competitors and governmental authorities, the impact of future developments related to the recently announced product recall, and various other factors subject to risks and uncertainties which could cause actual results to materially differ from those projected or implied in the forward-looking statements. Should any one or more of these risks or uncertainties materialize, or the underlying estimates or assumptions prove incorrect, actual results may vary significantly from those expressed in such forward-looking statements, and there can be no assurance that the forward-looking statements contained in this report will in fact occur.
| ITEM 1 | BUSINESS |
General
We are a leading developer, manufacturer and distributor of medical equipment for treating, diagnosing, and managing sleep-disordered breathing and other respiratory disorders. Sleep-disordered breathing, or SDB, includes obstructive sleep apnea, or OSA, and other respiratory disorders that occur during sleep. When we were formed in 1989, our primary purpose was to commercialize a treatment for OSA developed by Professor Colin Sullivan. This treatment, nasal Continuous Positive Airway Pressure, or CPAP, was the first successful noninvasive treatment for OSA. CPAP systems deliver pressurized air, typically through a nasal mask, to prevent collapse of the upper airway during sleep.
Since the development of CPAP, we have developed a number of innovative products for SDB and other respiratory disorders including airflow generators, diagnostic products, mask systems, headgear and other accessories. Our growth has been fuelled by geographic expansion, increased awareness of respiratory conditions as a significant health concern among physicians and patients, and our research and product development efforts.
We employ approximately 2,700 people and sell our products in over 68 countries through a combination of wholly owned subsidiaries and independent distributors.
- 2 -
Table of Contents
Our web site address is www.resmed.com. We make our periodic reports, together with any amendments, available on our web site, free of charge, as soon as reasonably practicable after we electronically file or furnish the reports with the Securities and Exchange Commission.
Corporate History
ResMed Inc., a Delaware corporation, was formed in March 1994 as the ultimate holding company for our Americas, Asia-Pacific and European operating subsidiaries. On June 1, 1995, we completed an initial public offering of common stock and on June 2, 1995 our common stock commenced trading on the NASDAQ National Market. On September 30, 1999 we transferred our principal public listing to the New York Stock Exchange, or NYSE, trading under the ticker symbol RMD. On November 25, 1999, we established a secondary listing of our common stock via Chess Depositary Instruments, or CDIs, on the Australian Stock Exchange, or ASX, also under the symbol RMD. Ten CDIs on the ASX represent one share of our common stock on the NYSE. On July 1, 2002, we converted our ASX listing status from a foreign exempt listing to a full listing.
Our Australian subsidiary, ResMed Holdings Limited, was originally organized in 1989 by Dr. Peter Farrell to acquire from Baxter Center for Medical Research Pty Limited, or Baxter, the rights to certain technology relating to CPAP treatment as well as Baxters existing CPAP device business. Baxter had sold CPAP devices in Australia since 1988, having acquired the rights to the technology in 1987.
Since formation we have acquired a number of operating businesses including:
| Name of Entity | Date of Acquisition | |
| Dieter W. Priess Medtechnik |
February 7, 1996 | |
| Premium Medical SARL |
June 12, 1996 | |
| Innovmedics Pte Ltd |
November 1, 1997 | |
| EINAR Egnell AB |
January 31, 2000 | |
| MAP Medizin Technologie GmbH |
February 16, 2001 | |
| Labhardt AG |
November 15, 2001 | |
| Servo Magnetics Inc. |
May 14, 2002 | |
| John Stark and Associates |
July 24, 2002 | |
| Respro Medical Company Limited |
July 2, 2003 | |
| Resprecare BV |
December 1, 2004 | |
| Hoefner Medizintechnik GmbH |
February 14, 2005 | |
| Saime SA |
May 19, 2005 | |
| Pulmomed Medizinisch-Technische Geräte GmbH |
July 1, 2005 | |
| PolarMed Holding AS |
December 1, 2005 | |
| Western Medical Marketing |
October 4, 2006 |
Segment Information
The Company believes that, given the single market focus of its operations solely in the sleep-disordered breathing sector of the respiratory medicine industry, and the inter-dependence of its products, the Company operates as a single operating segment. See Note 16 Segment Information of the Notes to Financial Statements (Part II, Item 8) for financial information regarding segment reporting. Financial information about our revenues from and assets located in foreign countries is also included in the notes to our consolidated financial statements.
The Market
Sleep is a complex neurological process that includes two distinct states: rapid eye movement, or REM, sleep and non-rapid eye movement, or non-REM, sleep. REM sleep, which is about 20-25% of
- 3 -
Table of Contents
total sleep experienced by adults, is characterized by a high level of brain activity, bursts of rapid eye movement, increased heart and respiration rates, and paralysis of many muscles. Non-REM sleep is subdivided into four stages that generally parallel sleep depth; stage 1 is the lightest and stage 4 is the deepest.
The upper airway has no rigid support and is held open by active contraction of upper airway muscles. Normally, during REM sleep and deeper levels of non-REM sleep, upper airway muscles relax and the airway narrows. Individuals with narrow upper airways or poor muscle tone are prone to temporary collapses of the upper airway during sleep, called apneas, and to near closures of the upper airway called hypopneas. These breathing irregularities result in a lowering of blood oxygen concentration, causing the central nervous system to react to the lack of oxygen or increased carbon dioxide and signaling the body to respond. Typically, the individual subconsciously arouses from sleep, causing the throat muscles to contract, opening the airway. After a few gasping breaths, blood oxygen levels increase and the individual can resume a deeper sleep until the cycle repeats itself. Sufferers of OSA typically experience ten or more such cycles per hour. While these awakenings greatly impair the quality of sleep, the individual is not normally aware of these disruptions. In addition, OSA has recently been recognized as a cause of hypertension and a significant co-morbidity for heart disease, stroke and diabetes.
Scientists estimate that one in five adults have some form of obstructive sleep apnea. In the United States alone, this represents approximately 43 million people. Despite the high prevalence of OSA, there is a general lack of awareness of OSA among both the medical community and the general public. It is estimated that less than 10% of those with OSA have been diagnosed or treated. Many healthcare professionals are often unable to diagnose OSA because they are unaware that such non-specific symptoms as excessive daytime sleepiness, snoring, hypertension and irritability are characteristic of OSA.
While OSA has been diagnosed in a broad cross-section of the population, it is predominant among middle-aged men and those who are obese, smoke, consume alcohol in excess or use muscle-relaxing and pain-killing drugs. A strong association has been discovered between OSA and a number of cardiovascular diseases. Recent studies have shown that SDB is present in approximately 80% of patients with drug-resistant hypertension, approximately 60% of stroke patients and approximately 50% of patients with congestive heart failure. More recently, studies have shown a connection between SDB and diabetes: recent studies indicate that SDB is independently associated with glucose intolerance and insulin resistance.
Sleep-Disordered Breathing and Obstructive Sleep Apnea
Sleep-disordered breathing encompasses all physiological processes that cause detrimental breathing patterns during sleep. Manifestations include OSA, central sleep apnea, or CSA, and hypoventilation syndromes that occur during sleep. Hypoventilation syndromes are generally associated with obesity, chronic obstructive lung disease and neuromuscular disease. OSA is the most common form of SDB.
Sleep fragmentation and the loss of the deeper levels of sleep caused by OSA can lead to excessive daytime sleepiness, reduced cognitive function, including memory loss and lack of concentration, depression and irritability. OSA sufferers also experience an increase in heart rate and an elevation of blood pressure during the cycle of apneas. Several studies indicate that the oxygen desaturation, increased heart rate and elevated blood pressure caused by OSA may be associated with increased risk of cardiovascular morbidity and mortality due to angina, stroke and heart attack. Patients with OSA have been shown to have impaired daytime performance in a variety of cognitive functions including problem solving, response speed and visual motor coordination, and studies have linked OSA to increased occurrences of traffic and workplace accidents.
- 4 -
Table of Contents
Generally, an individual seeking treatment for the symptoms of OSA is referred by a general practitioner to a specialist for further evaluation. The diagnosis of OSA typically requires monitoring the patient during sleep at either a sleep clinic or the patients home. During overnight testing, respiratory parameters and sleep patterns may be monitored, along with other vital signs such as heart rate and blood oxygen levels. Simpler tests, using devices such as our Apnealink, or our automatic positive airway pressure devices, monitor airflow during sleep, and use computer programs to analyze airflow patterns. These tests allow sleep clinicians to detect any sleep disturbances such as apneas, hypopneas or subconscious awakenings. We estimate that there are currently around 3,000 sleep clinics in the United States, a substantial portion of which are affiliated with hospitals. The number of sleep clinics has expanded significantly from approximately 100 such facilities in 1985.
Existing Therapies
Before 1981, the primary treatment for OSA was a tracheotomy, a surgical procedure to cut a hole in the patients windpipe to create a channel for airflow. Most recently, alternative treatments have involved either uvulopalatopharyngoplasty, or UPPP, in which surgery is performed on the upper airway to remove excess tissue and to streamline the shape of the airway, implanting a device to add support to the soft palate, or mandibular advancement, in which the lower jaw is moved forward to widen the patients airway. UPPP alone has a poor success rate; however, when performed in conjunction with multi-stage upper airway surgical procedures, a greater success rate has been claimed. These combined procedures, performed by highly specialized surgeons, are expensive and involve prolonged and often painful recovery periods.
CPAP, by contrast, is a non-invasive means of treating OSA. CPAP was first used as a treatment for OSA in 1980 by Dr. Colin Sullivan, the past Chairman of our Medical Advisory Board. CPAP systems were commercialized for treatment of OSA in the United States in the mid 1980s. Today, use of CPAP is generally acknowledged as the most effective and least invasive therapy for managing OSA.
During CPAP treatment, a patient sleeps with a nasal interface connected to a small portable airflow generator that delivers room air at a positive pressure. The patient breathes in air from the flow generator and breathes out through an exhaust port in the interface. Continuous air pressure applied in this manner acts as a pneumatic splint to keep the upper airway open and unobstructed. Interfaces include nasal masks and nasal pillows. Sometimes, when a patient leaks air through their mouth, a full-face mask may need to be used, rather than a nasal interface.
CPAP is not a cure and therefore, must be used on a nightly basis as long as treatment is required. Patient compliance has been a major factor in the efficacy of CPAP treatment. Early generations of CPAP units provided limited patient comfort and convenience. Patients experienced soreness from the repeated use of nasal masks and had difficulty falling asleep with the CPAP device operating at the prescribed pressure. In more recent years, product innovations to improve patient comfort and compliance have been developed. These include more comfortable patient interface systems; delay timers that gradually raise air pressure allowing the patient to fall asleep more easily; bilevel air flow generators, including Variable Positive Airway Pressure, or VPAP systems, which provide different air pressures for inhalation and exhalation; heated humidification systems to make the airflow more comfortable; and autotitration devices that reduce the average pressure delivered during the night.
Business Strategy
We believe that the SDB market will continue to grow in the future due to a number of factors including increasing awareness of OSA, improved understanding of the role of SDB treatment in the management of cardiac, neurologic, metabolic and related disorders, and an increase in home-based
- 5 -
Table of Contents
diagnosis. Our strategy for expanding our business operations and capitalizing on the growth of the SDB market consists of the following key elements:
Continue Product Development and Innovation. We are committed to ongoing innovation in developing products for the diagnosis and treatment of SDB. We have been a leading innovator of products designed to more effectively treat SDB, increase patient comfort and encourage compliance with prescribed therapy. For example, in 1999 we introduced the Mirage Full Face Mask. This mask contains an inflatable air pocket, which conforms to the patients facial contours, creating a more comfortable and better seal. In 2002, we introduced the AutoSet Spirit flow generator, our second-generation autotitrating device that adapts to the patients breathing patterns to more effectively treat OSA. In 2003, we introduced the Mirage Activa nasal mask, with active cushion technology to automatically seal mask leaks. In 2004, we introduced the Mirage Swift nasal pillows system, a less obtrusive, lightweight, and flexible alternative to nasal masks. In 2005, we introduced the S8 range of CPAP, a small flow generator with optional integrated humidification. In 2007, we launched several new patient interfaces including the Mirage Quattro, a full face mask that offers dual-wall cushion with spring air technology which accommodates movement during sleep, and the Mirage Liberty, which combines our nasal pillow technology in a full face mask product with a minimalist design. We believe that continued product development and innovation are key factors to our ongoing success. Approximately 12% of our employees are devoted to research and development activities. In fiscal year 2007, we invested $50.1 million, or 7% of our revenues, in research and development.
Expand Geographic Presence. We market our products in over 68 countries to sleep clinics, home healthcare dealers and third party payers. We intend to increase our sales and marketing efforts in our principal markets, as well as expand the depth of our presence in other geographic regions.
Increase Public and Clinical Awareness. We intend to continue to expand our existing promotional activities to increase awareness of SDB and our treatment alternatives. These promotional activities target the population with predisposition to SDB as well as primary care physicians and specialists, such as cardiologists, neurologists and pulmonologists. In addition, we also target special interest groups, including the National Stroke Association, the American Heart Association and the National Sleep Foundation.
During fiscal years 2007, 2006 and 2005, we donated $Nil, $0.8 million and $0.5 million, respectively, to the ResMed Foundation in the United States, and the ResMed Foundation in Australia, to further enhance research and awareness of SDB. The contributions to the Foundations reflect ResMeds commitment to medical research into sleep-disordered breathing, particularly the treatment of obstructive sleep apnea.
Expand into New Clinical Applications. We continually seek to identify new applications of our technology for significant unmet medical needs. Recent studies have established a clinical association between OSA and both stroke and congestive heart failure, and have recognized SDB as a cause of hypertension or high blood pressure. Research also indicates that SDB is independently associated with glucose intolerance and insulin resistance. We have developed a device for the treatment of Cheyne-Stokes breathing in patients with congestive heart failure. In addition, we maintain close working relationships with a number of prominent physicians to explore new medical applications for our products and technology. We have recently received Food and Drug Administration, or FDA, clearance and launched a new product in the United States for the treatment of respiratory insufficiency due to central sleep apnea, mixed apnea and periodic breathing, called the Adapt SV. The Adapt SV uses a technology known as adaptive servo-ventilation and was first made available to a select group of U.S. key opinion leader sites beginning in the third quarter of fiscal year 2006. Adaptive servo-ventilation, utilizes an advanced algorithm to calculate a patient-specific minute ventilation target and automatically adjusts pressure support to maintain the target. We believe this
- 6 -
Table of Contents
technology has allowed physicians to successfully treat complex breathing disorders in some patients who had previously tried and failed traditional positive airway pressure therapy.
Leverage the Experience of our Management Team. Our senior management team has extensive experience in the medical device industry in general, and in the field of SDB in particular. We intend to continue to leverage the experience and expertise of these individuals to maintain our innovative approach to the development of products and increase awareness of the serious medical problems caused by SDB.
Products
Our portfolio of products for the treatment of OSA and other forms of SDB includes airflow generators, diagnostic products, mask systems, headgear and other accessories.
Air Flow Generators
We produce CPAP, VPAP and AutoSet systems for the titration and treatment of SDB. The flow generator systems deliver positive airway pressure through a patient interface, either a small nasal mask, nasal pillows system, or full-face mask.
Our VPAP units deliver ultra-quiet, comfortable bilevel therapy. There are two preset pressures: a higher pressure as the patient breathes in, and a lower pressure as the patient breathes out. Breathing out against a lower pressure makes treatment more comfortable, particularly for patients who need high pressure levels or for those with impaired breathing ability.
AutoSet systems are based on a proprietary technology to monitor breathing and can also be used in the diagnosis, treatment and management of OSA. CPAP and VPAP flow generators accounted for approximately 52%, 52% and 49% of our net revenues in fiscal years 2007, 2006 and 2005, respectively.
- 7 -
Table of Contents
With the acquisition of Saime SA in May 2005, we increased our presence in the European homecare ventilation market. The VS and Elisée range of products are sophisticated, yet easy to use for physicians, clinicians and patients. We believe these devices complement our VPAP III, VPAP Adapt SV and Autoset CS2 for patients who need ventilatory assistance.
| VPAP PRODUCTS | DESCRIPTION | DATE OF COMMERCIAL INTRODUCTION |
||
| VPAP II |
Bilevel portable device providing different pressure levels for inhalation and exhalation, improved pressure switching and reduced noise output and spontaneous breath triggering. | March 1996 | ||
| COMFORT |
Bilevel device with limited features. | March 1996 | ||
| VPAP II ST |
Bilevel portable device with spontaneous and spontaneous/timed breath triggering modes of operation. | April 1996 | ||
| VPAP II STA |
Bilevel device with alarms. | August 1998 | ||
| VPAP MAX |
Bilevel ventilatory support system for the treatment of adult patients with respiratory insufficiency or respiratory failure. | November 1998 | ||
| Moritz S#* |
Bilevel portable device providing different pressure levels for inhalation and exhalation with integrated humidifier. | October 2001 | ||
| Moritz ST#* |
Bilevel ST device with spontaneous and spontaneous/timed breath triggering modes of operation, and with power failure alarms, system with integrated humidifier. | October 2001 | ||
| VPAP III |
Updated Bilevel portable device encompassing improved pressure synchronization, spontaneous breath triggering and reduced noise. | April 2003 | ||
| VPAP III ST |
Updated Bilevel ST portable device encompassing improved pressure synchronization, spontaneous and spontaneous/timed breath triggering modes of operation and reduced noise. | April 2003 | ||
| VPAP III STA |
An upgraded Bi-level device with alarm features. | August 2004 | ||
| Adapt SV |
The newest and most highly evolved bilevel device which uses adaptive servo-ventilation technology to treat patients with central sleep apnea, mixed apnea and periodic breathing. | March 2006 | ||
| VPAP Malibu |
Auto-adjusting bilevel device utilizing the smooth pressure waveform of the VPAP Adapt SV to achieve ultimate comfort for non-compliant CPAP users. | April 2007 | ||
* Not cleared for marketing in the United States
# Sold outside United States only
- 8 -
Table of Contents
| VENTILATION PRODUCTS | DESCRIPTION | DATE
OF COMMERCIAL INTRODUCTION |
||
| Helia 2*# |
Dual mode ventilator that combines volumetric and barometric ventilation modes. | August 1998 | ||
| Eole 3 XLS*# |
Ventilator device providing conventional volumetric ventilation through both controlled and assisted-controlled ventilation with etv functions. | December 1999 | ||
| VS Serena*# |
Bi-level ventilator providing all ventilation modes with two pressure levels. | June 2001 | ||
| VS Ultra*# |
Dual mode ventilator that combines volumetric and barometric ventilation from leakage to valve type with single or double limb circuit. | March 2002 | ||
| VS Integra*# |
Pressure support ventilator that combines pressure modes with leakage or valve ventilators. | March 2002 | ||
| Elisée 350*# |
Ventilator for use in Intensive Care Unit combining all conventional ventilation modes, diagnostic and monitoring functions. | December 2003 | ||
| Elisée 150*# |
Ventilator device that combines volumetric and barometric ventilation modes with single or double limb circuit. | June 2004 | ||
| Elisée 370*# |
Ventilator for use in Intensive Care Unit combining all conventional ventilation modes, diagnostic functions with external monitoring interface for ventilation loops. | September 2004 | ||
| Elisée 250*# |
Ventilator for use in transport and emergency situations. | April 2005 | ||
* Not cleared for marketing in the United States
# Sold outside United States only
- 9 -
Table of Contents
| AIR
FLOW GENERATORS |
DESCRIPTION | DATE OF COMMERCIAL INTRODUCTION |
||
| Automatic Positive Airway Pressure | ||||
| AutoSet CS*# |
Automatic ventilatory assistance device specifically designed to normalize ventilation in congestive heart failure patients with Cheyne Stokes respiration. | December 1998 | ||
| AutoSet T |
Autotitrating device, which continually adjusts CPAP treatment pressure based on patient airway resistance. | March 1999 | ||
| AutoSet Spirit |
Modular, autotitrating device with advanced compliance monitoring and optional integrated humidifier. | September 2001 | ||
| Magellan*# |
Autotitrating device using airway resistance measurement. | March 2003 | ||
| AutoSet Respond |
Autotitrating device with basic compliance monitoring and optional integrated humidifier. | September 2003 | ||
| AutoSet CS2*# |
Modular, automatic device specifically designed to normalize ventilation in congestive heart failure patients with Cheyne Stokes respiration. The device has an optional integrated humidifier. | August 2004 | ||
| CPAP |
||||
| Max II nCPAP*# |
CPAP device with or without integrated humidifier. Features low noise and reduced pressure swings. | April 1997 | ||
| Mini Max nCPAP*# |
CPAP device with integrated and attachable humidifier and low noise levels. | March 2000 | ||
| ResMed S6 series |
Quiet, compact CPAP device with various comfort features. | June 2000 | ||
| ResMed S7 series |
A CPAP device with optional integrated humidifier. | July 2002 | ||
| ResMed S8 Series |
A small CPAP device with optional integrated humidification. | June 2005 | ||
| C-Series Tango |
An entry level CPAP device with optional humidification | March 2007 | ||
* Not cleared for marketing in the United States
# Sold outside United States only
- 10 -
Table of Contents
Mask Systems and Diagnostic Products
Mask systems are one of the most important elements of SDB treatment systems. Masks are a primary determinant of patient comfort and as such may drive or impede patient compliance with therapy. We have been a consistent innovator in masks, improving patient comfort while minimizing size and weight. Masks, accessories, motors and diagnostic products accounted for approximately 48%, 48% and 51% of our net revenues in fiscal years 2007, 2006 and 2005, respectively.
| MASK PRODUCTS | DESCRIPTION | DATE OF COMMERCIAL INTRODUCTION |
||
| Mirage Mask | Proprietary mask design with a contoured nasal cushion that adjusts to patients facial contours. Quiet, light and low profile. | August 1997 | ||
| Ultra Mirage Mask | Advanced version of the Mirage system with reduced noise characteristics and improved forehead bridge. | June 2000 | ||
| Mirage Full Face Mask Series 2 | Mirage-based full-face mask system. Provides an effective method of applying ventilatory assist Noninvasive Positive Pressure Ventilation therapy. Can be used to address mouth- breathing problems in conventional bilevel or CPAP therapy. | October 2001 | ||
| Papillon Mask*# | Nasal mask with only four major parts, allows simplified handling for patients and distributors. | April 2002 | ||
| Mirage Vista Mask | Small nasal mask without forehead supports. | November 2002 | ||
| Ultra Mirage Full Face Mask | Full-face mask incorporating our latest adjustable forehead support technology. | August 2003 | ||
| Mirage Activa Mask | Nasal mask system utilizing Active Seal technology to mitigate leak and improve patient comfort. | October 2003 | ||
| Mirage Swift | A light and unobtrusive nasal cannula mask system. | August 2004 | ||
| Silent Papillon Mask*# | A low noise nasal mask with simplified assembly. | March 2005 | ||
| Hospital Full Face Mask | Disposable full face mask specifically designed for hospital use. | April 2005 | ||
| Hospital Nasal Mask | Disposable nasal mask specifically designed for hospital use. | April 2005 | ||
| Ultra Mirage II | Advanced version of the Ultra Mirage Nasal System with improved comfort and ease of fit through enhanced forehead pads and support. | July 2005 | ||
| Meridian Nasal Mask | A value line nasal mask that is simple yet comfortable. | February 2006 | ||
| Mirage Swift II | Improved design to reduce noise and airflow pattern. | April 2007 | ||
| Mirage Quattro | ResMeds fourth generation full face mask, delivering an individualized fit for over 95% of users. | April 2007 | ||
| Mirage Liberty |
A full face mask that seals individually at the mouth and nose. With less skin contact and an open field of vision, this unobtrusive mask feels light on the face. | May 2007 | ||
* Not cleared for marketing in the United States
# Sold outside United States only
- 11 -
Table of Contents
We market sleep recorders for the diagnosis and titration of SDB in sleep clinics and hospitals. These diagnostic systems record relevant respiratory and sleep data, which can be analyzed by a sleep specialist or physician who can then tailor an appropriate OSA treatment regimen for the patient.
| DIAGNOSTIC PRODUCTS | DESCRIPTION | DATE OF COMMERCIAL INTRODUCTION |
||
| Poly-MESAM Portable Diagnostic System*a# | Configurable cardio-respiratory polygraphy system up to 8 channels, includes ECG, thorax and abdomen belts, PLMS sensor. | February 1995 | ||
| MEPAL Diagnostic System*a # | Polysomnography system designed for use in the sleep laboratory. | February 1999 | ||
| Emblaa | Digital sleep recorder that provides comprehensive sleep diagnosis in a sleep laboratory. | October 1999 | ||
| Emblettaa | Pocket-size digital recorder that performs ambulatory sleep studies. | November 2000 | ||
| MEPAL mobil *a# Diagnostic System | Ambulatory polysomnography system. | March 2001 | ||
| ApneaLink (MicroMesam) | A portable Sleep Apnea screening device for use by sleep professionals and primary care physicians. | April 2004 | ||
| ApneaLink + Oximetry | A portable Sleep Apnea screening device with oximetry measurement | June 2007 | ||
* Not cleared for marketing in the United States
# Sold outside United States only
a Not manufactured by ResMed
- 12 -
Table of Contents
Accessories and Other Products
To enhance patient comfort, convenience and compliance, we market a variety of other products and accessories. These products include humidifiers, such as the HumidAire, H2i and H3i, which connect directly with the CPAP, VPAP and AutoSet flow generators to humidify and heat the air delivered to the patient. Their use helps prevent the drying of nasal passages that can cause discomfort. Other optional accessories include cold passover humidifiers, carry bags and breathing circuits. To assist those professionals diagnosing or managing the treatment of patients there are data communications and control products such as the ResLink, ResControl and ResControl II modules that facilitate the transfer of data and other information to and from the flow generators. Since the May 2002 acquisition of ResMed Motor Technologies Inc., we have also sold custom electric motors, primarily for use in data storage and aerospace applications, but we do not expect custom electric motor sales to contribute material revenues in the future.
Product Development and Clinical Trials
We have a strong track record in innovation in the sleep market. In 1989, we introduced our first CPAP device. Since then we have been committed to an ongoing program of product advancement and development. Currently, our product development efforts are focused on not only improving our current product offerings, but also expanding into new product applications.
In 1999, we introduced the AutoSet T flow generator, an autotitrating device that adapts to the patients breathing patterns to effectively prevent apneas. In 2001, we introduced our next generation autotitrating device, the AutoSet Spirit. The AutoSet Spirit is an autotitrating modular device with optional integrated humidifier. In 2003, we introduced the Activa nasal mask using our patented Active Cushion Technology, which automatically seals mask leaks. In 2004, we launched our Mirage Swift mask, a light and unobtrusive nasal cannula mask system. Also, in 2004 we launched an improved AutoSet CS 2 (outside the United States only) to treat congestive heart failure patients with significant central sleep apnea. In 2006, we launched the Adapt SV within the United States. This product is for the treatment of respiratory insufficiency due to central sleep apnea, mixed apnea and periodic breathing and uses a technology known as adaptive servo-ventilation.
We continually seek to identify new applications of our technology for significant unmet medical needs. SDB is associated with a number of symptoms beyond excessive daytime sleepiness and irritability. Recent studies have established a clinical association between SDB and hypertension, stroke, congestive heart failure and diabetes. We support clinical trials in the United States, Germany, France, the United Kingdom, Italy, Switzerland and Australia to develop new clinical applications for our technology.
We consult with physicians at major sleep centers throughout the world to identify technological trends in the treatment of SDB. Some of these physicians served on our Medical Advisory Board during fiscal year 2006. During fiscal year 2007, we reorganized our Medical Advisory Board into several regional advisory boards. New product ideas are also identified by our marketing staff, direct sales force, network of distributors, manufacturers representatives, customers and patients. Typically, our internal development staff then develops these ideas, where appropriate, into new products.
In fiscal years 2007, 2006 and 2005 we invested $50.1 million, $37.2 million and $30.0 million, respectively, on research and development.
Sales and Marketing
We currently market our products in over 68 countries using a network of distributors, independent manufacturers representatives and our direct sales force. We attempt to tailor our marketing approach
- 13 -
Table of Contents
to each national market, based on regional awareness of SDB as a health problem, physician referral patterns, consumer preferences and local reimbursement policies.
North America and Latin America. Our products are typically purchased by a home healthcare dealer who then sells the products to the patient. The decision to purchase our products, as opposed to those of our competitors, is made or influenced by one or more of the following individuals or organizations: the prescribing physician and his or her staff; the home healthcare dealer; the insurer and the patient. In the United States, our sales and marketing activities are conducted through a field sales organization made up of regional territory representatives, program development specialists and regional sales directors. Our U.S. field sales organization markets and sells products to home healthcare dealer branch locations throughout the United States.
We also promote and market our products directly to sleep clinics. Patients who are diagnosed with OSA and prescribed CPAP treatment are typically referred by the diagnosing sleep clinic to a home healthcare dealer to fill the prescription. The home healthcare dealer, in consultation with the referring physician, will assist the patient in selecting the equipment, fit the patient with the appropriate mask and set the flow generator pressure to the prescribed level.
In the United States, our sales employees are managed by the Chief Operating Officer Americas and Vice President of Sales. Sales in North and Latin America accounted for 53%, 52% and 51% of our net revenues for fiscal years 2007, 2006 and 2005, respectively.
Europe. We market our products in most major European countries. We have wholly-owned subsidiaries in Austria, Finland, France, Germany, Spain, Sweden, Norway, Netherlands, Switzerland and the United Kingdom that sell our products directly into those countries. We use independent distributors to sell our products in other areas of Europe. Distributors are selected in each country based on their knowledge of respiratory medicine and a commitment to SDB therapy. In each country in which we have a subsidiary, a local senior manager is responsible for direct national sales. In many countries in Europe, we sell our products to home healthcare dealers who then sell the products to the patients. In Germany, we also operate a home healthcare company, in which we provide products and services directly to patients, and receive reimbursement directly from third party payers.
Our European Chief Operating Officer is responsible for coordination of all European activities and, in conjunction with local management, the direct sales activity in Europe. Sales in Europe accounted for 39%, 39% and 41% of our total net revenues for fiscal years 2007, 2006 and 2005, respectively.
Asia Pacific. Marketing in Asia Pacific and the rest of the world is the responsibility of our Senior Vice President Sales & Marketing Asia Pacific. We have whollyowned subsidiaries in Australia, Hong Kong, Japan, Malaysia, New Zealand, Singapore, China and India that sell our products directly into those countries. We use a combination of our direct sales force and independent distributors in Australia and New Zealand, and use independent distributors to sell our products elsewhere in Asia Pacific. Sales in Asia Pacific and the rest of the world accounted for 8%, 9% and 8% of our total net revenues for the fiscal years 2007, 2006 and 2005, respectively.
Other Marketing Efforts. We continue to pursue other suitable opportunities with professional and healthcare associations to raise awareness of the co-morbidity of SDB in cardiovascular disease patients, including coronary artery disease, congestive heart failure, hypertension and stroke.
We also continue to work to raise awareness of SDB in diabetes. Current research is increasingly showing an independent association between OSA and type 2 diabetes. Accordingly, we initiated a study investigating the prevalence of OSA in the type 2 diabetic population. Due to the high
- 14 -
Table of Contents
prevalence of the SDB and type 2 diabetes, we are now actively supporting the American Association of Diabetes Educators and are in the process of setting up further initiatives to develop the SDB market in the diabetic population. ResMed is also reaching out to diabetes patients. Through our partnership with the American Diabetes Association, a sleep laboratory is now present at every Diabetes Expo meeting where patients have the opportunity to learn about diabetes self-management.
Manufacturing
Our principal manufacturing facility is located in Sydney, Australia and comprises a 155,000 square foot manufacturing facility. Our manufacturing operations consist primarily of assembly and testing of our flow generators, masks and accessories. Of the numerous raw materials, parts and components purchased for assembly of our therapeutic and diagnostic sleep disorder products, most are off-the-shelf items available from multiple vendors. We generally manufacture to our internal sales forecasts and fill orders as received. Over the last few years, the manufacturing processes have been transformed along lean manufacturing guidelines to flow lines staffed by dedicated teams. Each team is responsible for the manufacture and quality of their product group and decisions are based on performance and quality measures, including customer feedback.
Our quality management system is based upon the requirements of ISO 9001, ISO 13485, FDA Quality System Regulations for Medical Devices and the Medical Device Directive (93/42/EEC). Our Sydney, Australia and San Diego, California facilities are each accredited to ISO 9001 and ISO 13485. These two sites have third party audits conducted by the ISO certification bodies at regular intervals.
As part of the acquisition of Saime SA on May 19, 2005, we acquired a 7,000 square foot manufacturing facility in Paris, France. This facility is accredited to ISO 13485 and is primarily responsible for the assembly of the Saime brand of mechanical ventilators and associated accessories.
We also manufacture high-quality electric motors for our flow generator devices at our ResMed Motor Technologies Inc. facility. We have recently leased a larger site of 72,000 square feet at Chatsworth, California and moved our Resmed Motor Technology operations into this facility during the year ended June 30, 2007.
Third-Party Reimbursement
The cost of medical care in many of the countries in which we operate is funded in substantial part by government and private insurance programs. In Germany, we receive payments directly from these payers. Outside Germany, although we do not generally receive payments for our products directly from these payers, our success in major markets is dependent upon the ability of patients to obtain adequate reimbursement for our products.
In the United States, our products are purchased primarily by home healthcare dealers, hospitals or sleep clinics, which then invoice third-party payers directly for reimbursement. Domestic third-party payers include Medicare, Medicaid and corporate health insurance plans. These payers may deny reimbursement if they determine that a device is not used in accordance with cost-effective treatment methods, or is experimental, unnecessary or inappropriate. The long-term trend towards managed healthcare, or legislative proposals to reform healthcare, could control or significantly influence the purchase of healthcare services and products and could result in lower prices for our products. In some foreign markets, such as Spain, France and Germany, government reimbursement is currently available for purchase or rental of our products, however, subject to constraints such as price controls or unit sales limitations. In Australia and in some other foreign markets, there is currently limited or no reimbursement for devices that treat OSA.
- 15 -
Table of Contents
For example, the Medicare Prescription Drug, Improvement and Modernization Act of 2003 (the 2003 Act) reduced medical reimbursement for respiratory drugs and home oxygen to homecare providers and placed a freeze on current reimbursement levels for Durable Medical Equipment (DME) through 2008. As required by the 2003 Act, Medicare plans to implement competitive bidding of durable medical equipment in 10 of the largest Metropolitan Statistical Areas (MSA) by the end of 2007, and in 80 of the largest MSAs by the end of 2009. In addition, the U.S. Congress passed the Deficit Reduction Act of 2005 (2005 Act) in February 2006 which contained Medicare payment reductions for home oxygen equipment, and certain durable medical equipment classified by Medicare as capped rental equipment. In August 2006, the Centers for Medicare and Medicaid Services published a proposed regulation to implement the 2005 Act which could reduce Medicare reimbursement in 2007 for oxygen equipment. Additional reimbursement reductions for home oxygen were proposed in President Bushs Fiscal Year 2007 budget proposal, and could also be enacted into law. Both the federal government and state legislatures are considering options for containing growth in the Medicaid program.
Even though we do not file claims or bill governmental programs and other third-party payers directly for reimbursement for our products sold in the United States, we are still subject to laws and regulations relating to governmental programs, and any violation of these laws and regulations could result in civil and criminal penalties, including fines. In particular, the federal Anti-Kickback Law prohibits persons from knowingly and willfully soliciting, receiving, offering or providing remuneration, directly or indirectly, to induce either the referral of an individual, or the furnishing, recommending or arranging for a good or service, for which payment may be made under a Federal healthcare program such as the Medicare and Medicaid programs. The government has interpreted this law broadly to apply to the marketing and sales activities of manufacturers and distributors like us. Many states have adopted laws similar to the federal Anti-Kickback Law. We are also subject to other federal and state fraud laws applicable to payment from any third-party payer. These laws prohibit persons from knowingly and willfully filing false claims or executing a scheme to defraud any healthcare benefit program, including private third-party payers. These laws may apply to manufacturers and distributors who provide information on coverage, coding and reimbursement of their products to persons who bill third-party payers. We continuously strive to comply with these laws and believe that our arrangements do not violate these laws. Liability may still arise from the intentions or actions of the parties with whom we do business or from a different governmental agency interpretation of the laws.
Service and Warranty
We generally offer one-year and two-year limited warranties on our flow generator products. Warranties on mask systems are for 90 days. In most markets, we rely on our distributors to repair our products with parts supplied by us. In the United States, home healthcare dealers generally arrange shipment of products to our San Diego facility for repair.
We receive returns of our products from the field for various reasons. We believe that the level of returns experienced to date is consistent with levels typically experienced by manufacturers of similar devices. We provide for warranties and returns based on historical data.
Competition
The markets for our products are highly competitive. We believe that the principal competitive factors in all of our markets are product features, reliability and price. Customer support, reputation and efficient distribution are also important factors.
We compete on a market-by-market basis with various companies, some of which have greater financial, research, manufacturing and marketing resources than us. In the United States, our principal
- 16 -
Table of Contents
market, Respironics Inc.; DeVilbiss, a division of Sunrise Medical Inc.; Nellcor Puritan Bennett, a division of Covidien Ltd.; and Fisher & Paykel Healthcare Corporation Limited are the primary competitors for our products. Our principal European competitors are also Respironics, DeVilbiss, and Nellcor Puritan Bennett, as well as regional European manufacturers. The disparity between our resources and those of our competitors may increase as a result of the trend towards consolidation in the healthcare industry. In addition, our products compete with surgical procedures and dental appliances designed to treat OSA and other SDB related respiratory conditions. The development of new or innovative procedures or devices by others could result in our products becoming obsolete or noncompetitive, which would harm our revenues and financial condition.
Any product developed by us that gains regulatory clearance will have to compete for market acceptance and market share. An important factor in such competition may be the timing of market introduction of competitive products. Accordingly, the relative speed with which we can develop products, complete clinical testing and regulatory clearance processes and supply commercial quantities of the product to the market are important competitive factors. In addition, our ability to compete will continue to be dependent on the extent to which we are successful in protecting our patents and other intellectual property.
Patents and Proprietary Rights and Related Litigation
Through our subsidiaries ResMed Limited, MAP Medizin-Technologie GmbH, ResMed Motor Technologies Inc., and Saime SAS, we own or have licensed rights to 271 issued United States patents (including 82 design patents) and 376 issued foreign patents. In addition, there are 338 pending United States patent applications (including 113 design patent applications), 641 pending foreign patent applications, 610 registered foreign designs and 266 pending foreign designs. Some of these patents, patent applications and designs relate to significant aspects and features of our products.
Of our patents, 13 United States patents and 27 foreign patents are due to expire in the next five years, with 1 foreign patent due to expire in 2008, 2 in 2010, 16 in 2011, and 8 in 2012; and 7 United States patents in 2008, 2 United States patents in 2010, and 4 United States patents in 2011. We believe that the expiration of these patents will not have a material adverse impact on our competitive position.
We rely on a combination of patents, trade secrets, copyrights, trademarks and non-disclosure agreements to protect our proprietary technology and rights.
Litigation may be necessary to enforce patents issued to us, to protect our rights, or to defend third-party claims of infringement by us of the proprietary rights of others. Patent laws regarding the enforceability of patents vary from country to country. Therefore, there can be no assurance that patent issues will be uniformly resolved, or that local laws will provide us with consistent rights and benefits.
Government Regulations
Our products are subject to extensive regulation particularly as to safety, efficacy and adherence to FDA Quality System Regulation, and related manufacturing standards. Medical device products are subject to rigorous FDA and other governmental agency regulations in the United States and similar regulations of foreign agencies abroad. The FDA regulates the introduction, manufacture, advertising, labeling, packaging, marketing, distribution and record keeping for such products, in order to ensure that medical products distributed in the United States are safe and effective for their intended use. In addition, the FDA is authorized to establish special controls to provide reasonable assurance of the safety and effectiveness of most devices. Non-compliance with applicable requirements can result in import detentions, fines, civil penalties, injunctions, suspensions or losses of regulatory approvals,
- 17 -
Table of Contents
recall or seizure of products, operating restrictions, refusal of the government to approve product export applications or allow us to enter into supply contracts, and criminal prosecution.
The FDA requires that a manufacturer introducing a new medical device or a new indication for use of an existing medical device obtain either a Section 510(k) premarket notification clearance or a premarket approval, or PMA, before introducing it into the U.S. market. Our products currently marketed in the United States are marketed in reliance on 510(k) pre-marketing clearances as either Class I or Class II devices. The process of obtaining a Section 510(k) clearance generally requires the submission of performance data and often clinical data, which in some cases can be extensive, to demonstrate that the device is substantially equivalent to a device that was on the market before 1976 or to a device that has been found by the FDA to be substantially equivalent to such a pre-1976 device. As a result, FDA approval requirements may extend the development process for a considerable length of time. In addition, in some cases, the FDA may require additional review by an advisory panel, which can further lengthen the process. The PMA process, which is reserved for new devices that are not substantially equivalent to any predicate device and for high-risk devices or those that are used to support or sustain human life, may take several years and requires the submission of extensive performance and clinical information.
As a medical device manufacturer, all of our domestic and Australian manufacturing facilities are subject to inspection on a routine basis by the FDA. We believe that our design, manufacturing and quality control procedures are in substantial compliance with the FDAs regulatory requirements.
Sales of medical devices outside the United States are subject to regulatory requirements that vary widely from country to country. Approval for sale of our medical devices in Europe is through the CE mark process. Where appropriate, our products are CE marked to the European Unions Medical Device Directive. Under the CE marketing scheme, our products are classified as either Class I or Class II. Our devices are listed in Australia with the Therapeutic Goods Administration, or TGA, and in Canada with Health Canada.
Employees
As of June 30, 2007, we had approximately 2,700 employees or full time consultants, of which approximately 1,100 persons were employed in warehousing and manufacturing, 300 in research and development and 1,300 in sales, marketing and administration. Of our employees and consultants, approximately, 1,150 were located in Australia, 550 in the United States, 900 in Europe and 100 in Asia.
We believe that the success of our business will depend, in part, on our ability to attract and retain qualified personnel. None of our employees is covered by a collective bargaining agreement. We believe that our relationship with our employees is good.
| ITEM 1A | RISK FACTORS |
Before deciding to purchase, hold or sell our common stock, you should carefully consider the risks described below in addition to the other cautionary statements and risks described elsewhere, and the other information contained, in this Report and in our other filings with the SEC, including our subsequent reports on Forms 10-Q and 8-K. The risks and uncertainties described below are not the only ones we face. Additional risks and uncertainties not presently known to us or that we currently deem immaterial may also affect our business. If any of these known or unknown risks or uncertainties actually occurs with material adverse effects on us, our business, financial condition and results of operations could be seriously harmed. In that event, the market price for our common stock will likely decline, and you may lose all or part of your investment.
- 18 -
Table of Contents
Our inability to compete successfully in our markets may harm our business. The markets for our sleep-disordered breathing products are highly competitive and are characterized by frequent product improvements and evolving technology. Our ability to compete successfully depends, in part, on our ability to develop, manufacture and market innovative new products. The development of innovative new products by our competitors or the discovery of alternative treatments or potential cures for the conditions that our products treat could make our products noncompetitive or obsolete. Current competitors, new entrants, academics, and others are trying to develop new devices, alternative treatments or cures, and pharmaceutical solutions to the conditions our products treat.
Additionally, some of our competitors have greater financial, research and development, manufacturing and marketing resources than we do. The past several years have seen a trend towards consolidation in the healthcare industry and in the markets for our products. Industry consolidation could result in greater competition if our competitors combine their resources or if our competitors are acquired by other companies with greater resources than ours. This competition could increase pressure on us to reduce the selling prices of our products or could cause us to increase our spending on research and development and sales and marketing. If we are unable to develop innovative new products, maintain competitive pricing, and offer products that consumers perceive to be as reliable as those of our competitors, our sales or gross margins could decrease which would harm our business.
Our business depends on our ability to market effectively to dealers of home healthcare products and sleep clinics. We market our products primarily to home healthcare dealers and to sleep clinics that diagnose OSA and other sleep disorders. We believe that home healthcare dealers and sleep clinics play a significant role in determining which brand of product a patient will use. The success of our business depends on our ability to market effectively to home healthcare dealers and sleep clinics to ensure that our products are properly marketed and sold by these third parties.
We have limited resources to market to approximately the 3,000 U.S. sleep clinics and the more than 6,000 home healthcare dealer branch locations, most of which use, sell or recommend several brands of products. In addition, home healthcare dealers have experienced price pressures as government and third-party reimbursement has declined for home healthcare products, and home healthcare dealers are requiring price discounts and longer periods of time to pay for products purchased from us. We cannot assure you that sleep clinic physicians will continue to prescribe our products, or that home healthcare dealers or patients will not substitute competing products when a prescription specifying our products has been written.
We have expanded our marketing activities to target the population with a predisposition to sleep-disordered breathing as well as primary care physicians and various medical specialists. We cannot assure you that these marketing efforts will be successful in increasing awareness or sales of our products.
Any inability to market effectively our products outside the U.S. could impact our profitability. Approximately half our revenues are generated outside the U.S., in over 68 different countries. Many of these countries have unique regulatory, medical and business environments, which may adversely impact our ability to market our products. If we are unable to market effectively our products outside the U.S., our overall financial performance could decline.
Fluctuations in foreign currency exchange rates could result in declines in our reported sales and earnings. Since our international sales and a significant portion of our manufacturing costs are denominated in local currencies and not in U.S. dollars, our reported sales and earnings are subject to fluctuations in foreign exchange rates. We had foreign currency transaction losses in recent periods and may have further losses in the future. We expect that international sales will continue to be a significant portion of our business and that a significant portion of our manufacturing costs and research and development costs will continue to be denominated in Australian dollars.
- 19 -
Table of Contents
If we are unable to support our continued growth, our business could suffer. We have experienced rapid and substantial growth. As we continue to grow, the complexity of our operations increases, placing greater demands on our management. Our ability to manage our growth effectively depends on our ability to implement and improve our financial and management information systems on a timely basis and to effect other changes in our business including, the ability to monitor and improve manufacturing systems, information technology, and quality and regulatory compliance systems, among others. Unexpected difficulties during expansion, the failure to attract and retain qualified employees, the failure to successfully replace or upgrade our management information systems, the failure to manage costs or our inability to respond effectively to growth or plan for future expansion could cause our growth to stop. If we fail to manage effectively and efficiently our growth, our costs could increase faster than our revenues and our business could suffer.
If we fail to integrate our recent acquisitions with our operations, our business could suffer. During the past three fiscal years we have acquired Western Medical Marketing, PolarMed, Pulmomed, Saime, Hoefner and Resprecare. We continue to integrate these acquisitions into our operations. The integration requires significant efforts from each company and we may find it difficult to integrate the operations as personnel may leave and licensees, distributors or suppliers may terminate their arrangements or demand amended terms to these arrangements. Additionally, our management may have their attention diverted while trying to integrate these companies. If we are not able to successfully integrate the operations, we may not realize the anticipated benefits of these acquisitions.
We are subject to various risks relating to international activities that could affect our overall profitability. We manufacture substantially all of our products outside the U.S. and sell a significant portion of our products in non-U.S. markets. Sales outside North and Latin America accounted for approximately 47% and 48% of our net revenues in the years ended June 30, 2007 and 2006, respectively. We expect that sales within these areas will account for approximately 50% of our net revenues in the foreseeable future. Our sales outside of North America and our operations in Europe, Australia and Asia are subject to several difficulties and risks that are separate and distinct from those we face in our U.S. operations, including:
| | fluctuations in currency exchange rates; |
| | tariffs and other trade barriers; |
| | compliance with foreign medical device manufacturing regulations; |
| | difficulty in enforcing agreements and collect receivables through foreign legal systems; |
| | reduction in third party payer reimbursement for our products; |
| | inability to obtain import licenses; |
| | changes in trade policies and in U.S. and foreign tax policies; |
| | possible changes in export or import restrictions; and |
| | the modification or introduction of other governmental policies with potentially adverse effects. |
Government and private insurance plans may not adequately reimburse patients for our products, which could result in reductions in sales or selling prices for our products. Our ability to sell our products depends in large part on the extent to which reimbursement for the cost of our products will be available from government health administration authorities, private health insurers and other organizations. These third party payers are increasingly challenging the prices charged for medical products and services and can, without notice, deny coverage for treatments that may include the use of the Companys products. Therefore, even if a product is approved for
- 20 -
Table of Contents
marketing, we cannot assure you that reimbursement will be allowed for the product, that the reimbursement amount will be adequate or, that the reimbursement amount, even if initially adequate, will not subsequently be reduced. For example, in some markets, such as Spain, France and Germany, government reimbursement is currently available for purchase or rental of our products but is subject to constraints such as price controls or unit sales limitations. In other markets, such as Australia and the United Kingdom, there is currently limited or no reimbursement for devices that treat sleep-disordered breathing conditions. Additionally, future legislation or regulation concerning the healthcare industry or third party or governmental coverage and reimbursement, particularly legislation or regulation limiting consumers reimbursement rights, may harm our business.
As we continue to develop new products, those products will generally not qualify for reimbursement, if at all, until they are approved for marketing. In the United States, we sell our products primarily to home healthcare dealers and to sleep clinics. We do not file claims and bill governmental programs or other third party payers directly for reimbursement for our products. However, we are still subject to laws and regulations relating to governmental reimbursement programs, particularly Medicaid and Medicare.
In addition to reimbursement for our products, our customers depend in part on reimbursement by government and private health insurers for other products. Any proposed reductions in reimbursement, if they occur, may have a material impact on our customers. Any material impact on our customers may indirectly affect our sales to those customers, or the collectibility of receivables we have from those customers.
Failure to comply with anti-kickback and fraud regulations could result in substantial penalties and changes in our business operations. In particular, the federal Anti-Kickback Law prohibits persons from knowingly and willfully soliciting, receiving, offering or providing remuneration, directly or indirectly, to induce either the referral of an individual, or the furnishing, recommending or arranging for a good or service, for which payment may be made under a federal healthcare program such as the Medicare and Medicaid programs. The U.S. government has interpreted this law broadly to apply to the marketing and sales activities of manufacturers and distributors like us. Many states and other governments have adopted laws similar to the federal Anti-Kickback Law. We are also subject to other federal and state fraud laws applicable to payment from any third party payer. These laws prohibit persons from knowingly and willfully filing false claims or executing a scheme to defraud any healthcare benefit program, including private third party payers. These laws may apply to manufacturers and distributors who provide information on coverage, coding, and reimbursement of their products to persons who do bill third party payers. Any violation of these laws and regulations could result in civil and criminal penalties (including fines), increased legal expenses and exclusions from governmental reimbursement programs, all of which could have a material adverse effect upon our business, financial conditions and results of operations.
Complying with Food and Drug Administration, or FDA, and other regulations is an expensive and time-consuming process, and any failure to comply could have a materially adverse effect on the Companys business, financial condition, or results of operations. We are subject to various federal, state, local and international regulations regarding our business activities. Failure to comply with these regulations could result in, among other things, recalls of our products, substantial fines and criminal charges against us or against our employees. Furthermore, our products could be subject to recall if the FDA or we determine, for any reason, that our products are not safe or effective. Any recall or other regulatory action could increase our costs, damage our reputation, affect our ability to supply customers with the quantity of products they require and materially affect our operating results. For example, in April 2007 we announced a worldwide voluntary product recall of approximately 300,000 of our S8 flow generators manufactured between July 2004 and May 2006. We have determined that there is a remote potential for a short circuit in the power connector. In only seven cases worldwide, device failures have led to thermal damage to the device, with a remote
- 21 -
Table of Contents
potential to ignite material external to the device. To date, no significant property damage or patient injury has been reported. The estimated cost of this action is $59.7 million, which we recognized as an expense in the year ended June 30, 2007. We cannot assure you that this will be the total cost for the recall or that the total cost will not significantly exceed our estimates. Moreover, we cannot predict the effect this recall and the negative publicity associated with the recall will have on our reputation among physicians and customers. Our results of operations could be severely impacted if we have failed to accurately estimate the costs of this product recall or if physicians and customers cease to recommend and purchase our products as a result of this product recall.
Product sales, introductions or modifications may be delayed or canceled as a result of FDA regulations or similar foreign regulations, which could cause our sales and profits to decline. Before we can market or sell a new medical device in the United States, we must obtain FDA clearance, which can be a lengthy and time-consuming process. We generally receive clearance from the FDA to market our products in the United States under Section 510(k) of the Federal Food, Drug, and Cosmetic Act or our products are exempt from the Section 510(k) clearance process. We have modified some of our Section 510(k) approved products without submitting new Section 510(k) notices, which we do not believe were required. However, if the FDA disagrees with us and requires us to submit new Section 510(k) notifications for modifications to our existing products, we may be required to stop marketing the products while the FDA reviews the Section 510(k) notification.
Any new product introduction or existing product modification could be subjected to a lengthier, more rigorous FDA examination process. For example, in certain cases we may need to conduct clinical trials of a new product before submitting a 510(k) notice. Additionally, we may be required to obtain premarket approvals for our products. The requirements of these more rigorous processes could delay product introductions and increase the costs associated with FDA compliance. Marketing and sale of our products outside the United States are also subject to regulatory clearances and approvals, and if we fail to obtain these regulatory approvals, our sales could suffer.
We cannot assure you that any new products we develop will receive required regulatory approvals from U.S. or foreign regulatory agencies.
The Company is subject to substantial regulation related to quality standards applicable to its manufacturing and quality processes. Failure by the Company to comply with these standards could have an adverse effect on the Companys business, financial condition, or results of operations. The FDA regulates the approval, manufacturing, and sales and marketing of many of the Companys products in the U.S. Significant government regulation also exists in Canada, Japan, Europe, and other countries in which the Company conducts business. As a device manufacturer, the Company is required to register with the FDA and is subject to periodic inspection by the FDA for compliance with the FDAs Quality System Regulation (QSR) requirements, which require manufacturers of medical devices to adhere to certain regulations, including testing, quality control and documentation procedures. In addition, the federal Medical Device Reporting regulations require the Company to provide information to the FDA whenever there is evidence that reasonably suggests that a device may have caused or contributed to a death or serious injury or, if a malfunction were to occur, could cause or contribute to a death or serious injury. Compliance with applicable regulatory requirements is subject to continual review and is rigorously monitored through periodic inspections by the FDA. In the European Community, the Company is required to maintain certain ISO certifications in order to sell its products and must undergo periodic inspections by notified bodies to obtain and maintain these certifications. Failure to comply with current governmental regulations and quality assurance guidelines could lead to temporary manufacturing shutdowns, product recalls or related field actions, product shortages or delays in product manufacturing. Efficacy or safety concerns, an increase in trends of adverse events in the marketplace, and/or manufacturing quality issues with respect to the Companys products could lead to product recalls or related field actions, withdrawals, and/or declining sales.
- 22 -
Table of Contents
Off-label marketing of our products could result in substantial penalties. Clearance under Section 510(k) only permits us to market our products for the uses indicated on the labeling cleared by the FDA. We may request additional label indications for our current products, and the FDA may deny those requests outright, require additional expensive clinical data to support any additional indications or impose limitations on the intended use of any cleared products as a condition of clearance. If the FDA determines that we have marketed our products for off-label use, we could be subject to fines, injunctions or other penalties.
Disruptions in the supply of components from our single source suppliers could result in a significant reduction in sales and profitability. We purchase uniquely configured components for our devices from various suppliers, including some who are single-source suppliers for us. We cannot assure you that a replacement supplier would be able to configure its components for our devices on a timely basis or, in the alternative, that we would be able to reconfigure our devices to integrate the replacement part.
A reduction or halt in supply while a replacement supplier reconfigures its components, or while we reconfigure our devices for the replacement part, would limit our ability to manufacture our devices, which could result in a significant reduction in sales and profitability. We cannot assure you that our inventories would be adequate to meet our production needs during any prolonged interruption of supply.
Our intellectual property may not protect our products, and/or our products may infringe on the intellectual property rights of third parties. We rely on a combination of patents, trade secrets and non-disclosure agreements to protect our intellectual property. Our success depends, in part, on our ability to obtain and maintain United States and foreign patent protection for our products, their uses and our processes to preserve our trade secrets and to operate without infringing on the proprietary rights of third parties. We have a number of pending patent applications, and we do not know whether any patents will issue from any of these applications. We do not know whether any of the claims in our issued patents or pending applications will provide us with any significant protection against competitive products or otherwise be commercially valuable. Legal standards regarding the validity of patents and the proper scope of their claims are still evolving, and there is no consistent law or policy regarding the valid breadth of claims. Additionally, there may be third party patents, patent applications and other intellectual property relevant to our products and technology which are not known to us and that block or compete with our products.
We face the risks that:
| | third parties will infringe our intellectual property rights; |
| | our non-disclosure agreements will be breached; |
| | we will not have adequate remedies for infringement; |
| | our trade secrets will become known to or independently developed by our competitors; or |
| | third parties will be issued patents that may prevent the sale of our products or require us to license and pay fees or royalties in order for us to be able to market some of our products. |
Litigation may be necessary to enforce patents issued to us, to protect our proprietary rights, or to defend third party claims that we have infringed upon proprietary rights of others. For example, we are currently appealing the decision of a court in Germany that entered judgment in favor of certain plaintiffs that had claimed they should be listed as co-inventors on two of our German patent applications. The defense and prosecution of patent claims, including these pending claims, as well as participation in other inter-party proceedings, can be expensive and time consuming, even in those
- 23 -
Table of Contents
instances in which the outcome is favorable to us. If the outcome of any litigation or proceeding brought against us were adverse, we could be subject to significant liabilities to third parties, could be required to obtain licenses from third parties, could be forced to design around the patents at issue or could be required to cease sales of the affected products. A license may not be available at all or on commercially viable terms, and we may not be able to redesign our products to avoid infringement. Additionally, the laws regarding the enforceability of patents vary from country to country, and we cannot assure you that any patent issues we face will be uniformly resolved, or that local laws will provide us with consistent rights and benefits.
We are subject to potential product liability claims that may exceed the scope and amount of our insurance coverage, which would expose us to liability for uninsured claims. We are subject to potential product liability claims as a result of the design, manufacture and marketing of medical devices. In April 2007, we announced a worldwide voluntary product recall of approximately 300,000 of our S8 flow generators manufactured between July 2004 and May 2006. We have determined that there is a remote potential for a short circuit in the power connector which can cause the device to fail. In only seven cases worldwide, device failures have led to thermal damage to the device, with a remote potential to ignite material external to the device. To date, no significant property damage or patient injury has been reported. However, we would likely be subject to product liability claims should any of these devices malfunction, resulting in injury to a patient or damage to property. Any product liability claim brought against us, with or without merit, could result in the increase of our product liability insurance rates. In addition, we would have to pay any amount awarded by a court in excess of our policy limits. Our insurance policies have various exclusions, and thus we may be subject to a product liability claim for which we have no insurance coverage, in which case, we may have to pay the entire amount of any award. We cannot assure you that our insurance coverage will be adequate or that all claims brought against us will be covered by our insurance. Insurance varies in cost and can be difficult to obtain, and we cannot assure you that we will be able to obtain insurance in the future on terms acceptable to us or at all. A successful product liability claim brought against us in excess of our insurance coverage, if any, may require us to pay substantial amounts, which could harm our business.
We are subject to tax audits by various tax authorities in many jurisdictions. From time to time we may be audited by the tax authorities and are still subject to an ongoing German tax audit. Any final assessment resulting from this audit could result in material changes to our past or future taxable income, tax payable or deferred tax assets, and could require us to pay penalties and interest that could materially adversely affect our financial results.
Our quarterly operating results are subject to fluctuation for a variety of reasons. Our operating results have, from time to time, fluctuated on a quarterly basis and may be subject to similar fluctuations in the future. These fluctuations may result from a number of factors, including:
| | the introduction of new products by us or our competitors; |
| | the geographic mix of product sales; |
| | the success of our marketing efforts in new regions; |
| | changes in third party reimbursement; |
| | timing of regulatory clearances and approvals; |
| | timing of orders by distributors; |
| | expenditures incurred for research and development; |
| | competitive pricing in different regions; |
- 24 -
Table of Contents
| | seasonality; |
| | the cost and effect of promotional and marketing programs; |
| | the effect of foreign currency transaction gains or losses; and |
| | other activities of our competitors. |
Fluctuations in our quarterly operating results may cause the market price of our common stock to fluctuate.
If a natural or man-made disaster strikes our manufacturing facilities, we will be unable to manufacture our products for a substantial amount of time and our sales and profitability will decline. Our facilities and the manufacturing equipment we use to produce our products would be costly to replace and could require substantial lead-time to repair or replace. The facilities may be affected by natural or man-made disasters and in the event they were affected by a disaster, we would be forced to rely on third party manufacturers. Although we believe we possess adequate insurance for damage to our property and the disruption of our business from casualties, such insurance may not be sufficient to cover all of our potential losses and may not continue to be available to us on acceptable terms, or at all.
Delaware law, provisions in our charter and our shareholder rights plan could make it difficult for another company to acquire us. Provisions of our certificate of incorporation may have the effect of delaying or preventing changes in control or management which might be beneficial to us or our security holders. In particular, our Board of Directors is divided into three classes, serving for staggered three-year terms. Because of this classification it will require at least two annual meetings to elect directors constituting a majority of our Board of Directors.
Additionally, our Board of Directors has the authority to issue up to 2,000,000 shares of preferred stock and to determine the price, rights, preferences, privileges and restrictions, including voting rights, of those shares without further vote or action by the stockholders. The rights of the holders of our common stock will be subject to, and may be adversely affected by, the rights of the holders of any preferred stock that may be issued in the future. The issuance of preferred stock may have the effect of delaying, deferring or preventing a change in control, may discourage bids for our common stock at a premium over the market price of our common stock and may adversely affect the market price of our common stock and the voting and other rights of the holders of our common stock.
You may not be able to enforce the judgments of U.S. courts against some of our assets or officers and directors. A substantial portion of our assets are located outside the United States. Additionally, two of our eight directors and three of our seven executive officers reside outside the United States, along with all or a substantial portion of the assets of these persons. As a result, it may not be possible for investors to enforce judgments of U.S. courts relating to any liabilities under U.S. securities laws against our assets, those persons or their assets. In addition, we have been advised by our Australian counsel that some doubt exists as to the ability of investors to pursue claims based on U.S. securities laws against these assets or these persons in Australian courts.
| ITEM 1B | UNRESOLVED STAFF COMMENTS |
We have received no written comments regarding our periodic or current reports from the staff of the Securities and Exchange Commission that were issued 180 days or more preceding the end of our fiscal year 2007 that remain unresolved.
- 25 -
Table of Contents
| ITEM 2 | PROPERTIES |
Our principal executive offices and U.S. distribution facilities, consisting of approximately 144,000 square feet, are located in Poway (North San Diego County), California in a building we own. During the year ended June 30, 2007, we completed the construction of our new research and development and office facilities at our existing site in Norwest, Sydney, Australia, which consists of approximately 69,000 square feet. We own our principal manufacturing facility consisting of a 155,000 square foot complex at this same Norwest site in Sydney, Australia. During the year ended June 30, 2007, we commenced an extension to this manufacturing facility, which we expect to complete within the next fiscal year. We lease a 72,000 square foot facility for manufacture of electronic motors in Chatsworth, California. On July 7, 2005, we purchased a 9.78-acre parcel of land in San Diego for $21.0 million. The new location at Kearney Mesa, San Diego will allow us to develop a new corporate headquarters. We commenced construction of our new corporate headquarters during 2007 and we expect to complete the project in March 2009.
Sales and warehousing facilities are either leased or owned in South Carolina and Oregon, U.S.A.; Abingdon, England; Munich, Germany; Bremen, Germany; Hochstadt, Germany; Lyon, France; Paris, France; Basel, Switzerland; Trollhaettan, Sweden; Villach and Vienna, Austria; Helsinki, Finland; Den Haag, Netherlands; Oslo, Norway; Kowloon, Hong Kong; Auckland, New Zealand and Singapore.
| ITEM 3 | LEGAL PROCEEDINGS |
In the normal course of business, we are subject to routine litigation incidental to our business. While the results of this litigation cannot be predicted with certainty, we believe that their final outcome will not have a material adverse effect on our consolidated financial statements taken as a whole.
During September and October 2004, we began receiving tax assessment notices for the audit of one of our German subsidiaries by the German tax authorities for the years 1996 through 1998. Certain of these adjustments are being contested and appealed to the German tax authority office. We believe no additional provision is necessary for any tax adjustment that may result from the tax audit. However, the outcome of the audit cannot be predicted with certainty. Should any tax audit issues be resolved in a manner not consistent with managements expectations, we could be required to adjust our provision for income tax in the period of resolution.
In December 2002, three former contractors of our subsidiary MAP Medizin-Technologie GmbH initiated proceedings in Munich 1 Regional Court (Proceedings No. 7 O 23286/02), petitioning the Court for a declaration of inventorship with respect to MAP German Patent Applications identified as No. 100 31 079 and 101 92 802.5 and European Patent Application No. EP 01 967 819.7. On March 10, 2005, the Court entered judgment in favor of the plaintiffs, finding that they should be identified as co-inventors in place of certain individual defendants. In April 2005, MAP filed an appeal of that decision. We do not expect the outcome of this litigation to have an adverse material effect on our consolidated financial statements.
In March 2006, an Australian university made a demand that ResMed pay extra royalties pursuant to a current patent license agreement. ResMed rejected the demand and have informed the university that it does not consider the claim to have merit. In February 2007, the university commenced legal action in the Federal Court of Australia to pursue its claim against ResMed. ResMed is vigorously defending its position and does not expect the outcome of this claim to have an adverse material effect on ResMeds condensed consolidated financial statements.
| ITEM 4 | SUBMISSION OF MATTERS TO A VOTE OF SECURITY HOLDERS |
None.
- 26 -
Table of Contents
| ITEM 5 | MARKET FOR REGISTRANTS COMMON EQUITY, RELATED STOCKHOLDER MATTERS AND ISSUER PURCHASES OF EQUITY SECURITIES |
Our common stock is traded on the New York Stock Exchange (NYSE) under the symbol RMD. The following table sets forth for the fiscal periods indicated the high and low closing prices for the common stock as reported by the New York Stock Exchange.
| 2007 | 2006 | |||||||||||
| High | Low | High | Low | |||||||||
| Quarter One, ended September 30 |
$ | 48.40 | $ | 38.52 | $ | 40.03 | $ | 32.21 | ||||
| Quarter Two, ended December 31 |
51.08 | 39.53 | 42.72 | 37.01 | ||||||||
| Quarter Three, ended March 31 |
54.26 | 45.18 | 44.31 | 36.86 | ||||||||
| Quarter Four, ended June 30 |
51.41 | 41.25 | 48.50 | 41.76 | ||||||||
As of August 17, 2007, there were 48 holders of record of our common stock. We have not paid any cash dividends on our common stock since the initial public offering of our common stock and we do not currently intend to pay cash dividends in the foreseeable future. We anticipate that all of our earnings and other cash resources, if any, will be retained for the operation and expansion of our business and for general corporate purposes.
All share and per share information has been adjusted to reflect the two-for-one stock split effected in the form of a 100% stock dividend that was declared on August 10, 2005 and distributed on September 30, 2005.
Sale of Unregistered Securities
During fiscal year 2006, and pursuant to the Indenture dated June 20, 2001 between us and American Stock Transfer & Trust Company, as trustee, holders of all of our 4% Convertible Subordinated Notes (the Notes) due 2006 converted the Notes into an aggregate of approximately 3,737,593 shares of our common stock, par value $0.004, based on a conversion price of $30.30 per share. The shares of common stock were issued solely to existing security holders upon conversion of the Notes pursuant to the exemption from registration provided under Section 3(a)(9) of the Securities Exchange Act 1993, as amended. We did not pay or give, directly or indirectly, any commission or other remuneration for soliciting such conversion.
- 27 -
Table of Contents
Purchases of Equity Securities
The following table summarizes purchases by us of our common stock during the year ended June 30, 2007:
| Period | Total of Shares |
Average Price Paid per Share |
Total Number of Shares Purchased as Part of Publicly Announced Plans or Programs(1) |
Maximum Number of Shares that May yet be Purchased Under the Plans or Programs(1) |
||||||
| Opening Balance at July 1, 2006 |
2,254,918 | $ | 18.36 | 2,254,918 | 5,745,082 | |||||
| July 2006 |
Nil | |||||||||
| August 2006 |
Nil | |||||||||
| September 2006 |
Nil | |||||||||
| October 2006 |
Nil | |||||||||
| November 2006 |
Nil | |||||||||
| December 2006 |
Nil | |||||||||
| January 2007 |
Nil | |||||||||
| February 2007 |
Nil | |||||||||
| March 2007 |
Nil | |||||||||
| April 2007 |
Nil | |||||||||
| May 2007 |
50,000 | $ | 41.83 | 50,000 | (50,000 | ) | ||||
| June 2007 |
Nil | |||||||||
| Total to June 30, 2007 |
2,304,918 | $ | 18.87 | 2,304,918 | 5,695,082 | |||||
(1) On June 6, 2002, the Board of Directors authorized us to repurchase up to 8.0 million shares of our outstanding common stock. There is no expiration date for the repurchase of these shares. For the years ended June 30, 2007 and 2006, we repurchased 50,000 and Nil shares at a cost of $2.1 million and $Nil, respectively. At June 30, 2007, we have repurchased a total of 2,304,918 shares at a cost of $43.5 million. We may continue to repurchase shares of our common stock for cash in the open market, or in negotiated or block transactions, from time to time as market and business conditions warrant.
- 28 -
Table of Contents
| ITEM 6 | SELECTED FINANCIAL DATA |
The following table summarizes certain selected consolidated financial data for, and as of the end of, each of the fiscal years in the five-year period ended June 30, 2007. The data set forth below should be read in conjunction with the Managements Discussion and Analysis of Financial Condition and Results of Operations and our Consolidated Financial Statements and related Notes included elsewhere in this Report. The consolidated statements of operations data for the years ended June 30, 2007, 2006 and 2005 and the balance sheet data as of June 30, 2007 and 2006 are derived from our audited consolidated financial statements included elsewhere in this Report. The consolidated statements of operations data for the years ended June 30, 2004 and 2003 and the balance sheet data as of June 30, 2005, 2004 and 2003 are derived from our audited consolidated financial statements not included herein. Historical results are not necessarily indicative of the results to be expected in the future, and the results for the years presented should not be considered indicative of our future results of operations.
| Consolidated Statement of Income Data: | Years Ended June 30 | |||||||||||||||||||
| (In thousands, except per share data) | 2007 | 2006 | 2005 | 2004 | 2003 | |||||||||||||||
| Net revenues |
$ | 716,332 | $ | 606,996 | $ | 425,505 | $ | 339,338 | $ | 273,570 | ||||||||||
| Cost of sales |
272,140 | 230,101 | 150,645 | 122,602 | 100,483 | |||||||||||||||
| Voluntary product recall expenses |
59,700 | - | - | - | - | |||||||||||||||
| Gross profit | 384,492 | 376,895 | 274,860 | 216,736 | 173,087 | |||||||||||||||
| Selling, general and administrative expenses | 237,326 | 200,168 | 135,703 | 104,706 | 85,313 | |||||||||||||||
| Research and development expenses | 50,106 | 37,216 | 30,014 | 26,169 | 20,534 | |||||||||||||||
| Donations to research foundations | - | 760 | 500 | 500 | - | |||||||||||||||
| In-process research and development charge | - | - | 5,268 | - | - | |||||||||||||||
| Amortization of acquired intangible assets | 6,897 | 6,327 | 870 | - | - | |||||||||||||||
| Restructuring expenses | - | 1,124 | 5,152 | - | - | |||||||||||||||
| Total operating expenses | 294,329 | 245,595 | 177,507 | 131,375 | 105,847 | |||||||||||||||
| Income from operations | 90,163 | 131,300 | 97,353 | 85,361 | 67,240 | |||||||||||||||
| Other income (expenses): | ||||||||||||||||||||
| Interest income (expense), net | 6,477 | 1,320 | (808 | ) | (1,683 | ) | (2,549 | ) | ||||||||||||
| Other, net | 1,333 | 774 | 81 | 990 | 1,907 | |||||||||||||||
| Gain on extinguishment of debt | - | - | - | - | 529 | |||||||||||||||
| Total other income (expenses) |
7,810 | 2,094 | (727 | ) | (693 | ) | (113 | ) | ||||||||||||
| Income before income taxes | 97,973 | 133,394 | 96,626 | 84,668 | 67,127 | |||||||||||||||
| Income taxes | (31,671 | ) | (45,183 | ) | (31,841 | ) | (27,384 | ) | (21,398 | ) | ||||||||||
| Net income |
$ | 66,302 | $ | 88,211 | $ | 64,785 | $ | 57,284 | $ | 45,729 | ||||||||||
| Basic earnings per share | $ | 0.86 | $ | 1.22 | $ | 0.94 | $ | 0.85 | $ | 0.69 | ||||||||||
| Diluted earnings per share | $ | 0.85 | $ | 1.16 | $ | 0.91 | $ | 0.82 | $ | 0.66 | ||||||||||
| Weighted average: | ||||||||||||||||||||
| Basic shares outstanding |
76,709 | 72,307 | 68,643 | 67,389 | 66,108 | |||||||||||||||
| Diluted shares outstanding |
78,253 | 77,162 | 74,942 | 70,251 | 68,878 | |||||||||||||||
- 29 -
Table of Contents
All share and per share information has been adjusted to reflect the two-for-one stock split effected in the form of a 100% stock dividend that was declared on August 10, 2005 and distributed on September 30, 2005.
| Consolidated Balance Sheet Data: | As of June 30 | ||||||||||||||
| (In thousands) | 2007 | 2006 | 2005 | 2004 | 2003 | ||||||||||
| Working capital |
$ | 466,396 | $ | 381,284 | $ | 141,659 | $ | 222,230 | $ | 191,322 | |||||
| Total assets |
1,252,042 | 1,012,921 | 774,146 | 549,151 | 459,595 | ||||||||||
| Long-term debt, less current maturities |
87,648 | 116,212 | 58,934 | 113,250 | 113,250 | ||||||||||
| Total stockholders equity |
931,222 | 738,148 | 474,065 | 361,499 | 286,433 | ||||||||||
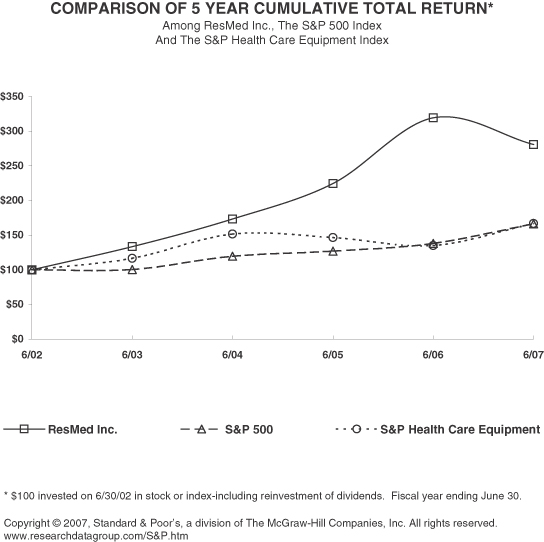
- 30 -
Table of Contents
| ITEM 7 | MANAGEMENTS DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS |
Overview
Managements discussion and analysis (MD&A) of financial condition and results of operations is intended to help the reader understand the results of operations and financial condition of Resmed Inc. MD&A is provided as a supplement to, and should be read in conjunction with selected financial data and consolidated financial statements and notes, included herein.
We are a leading developer, manufacturer and distributor of medical equipment for treating, diagnosing, and managing sleep-disordered breathing and other respiratory disorders. Sleep-disordered breathing, or SDB, includes obstructive sleep apnea, or OSA, and other respiratory disorders that occur during sleep. When we were formed in 1989, our primary purpose was to commercialize a treatment for OSA developed by Professor Colin Sullivan. This treatment, nasal Continuous Positive Airway Pressure, or CPAP, was the first successful noninvasive treatment for OSA. CPAP systems deliver pressurized air, typically through a nasal mask, to prevent collapse of the upper airway during sleep.
We have invested significant resources in research and development and product enhancement. Since the development of CPAP, we have developed a number of innovative products for SDB and other respiratory disorders including airflow generators, diagnostic products, mask systems, headgear and other accessories. Our growth has been fuelled by geographic expansion, increased awareness of respiratory conditions as a significant health concern among physicians and patients, and our research and product development effort.
We currently employ approximately 2,700 people and market our products in over 68 countries using a network of distributors, independent manufacturers representatives and our direct sales force. We market our products primarily to home health care dealers and sleep clinics. We attempt to tailor our marketing approach to each national market, based on regional awareness of SDB as a health problem, physician referral patterns, consumer preferences and local reimbursement policies.
Our principal manufacturing facility is located in Sydney, Australia, and we have additional manufacturing facilities in Combs La Ville, France and Chatsworth, California. Our manufacturing operations consist primarily of assembly and testing of our flow generators, masks and accessories. Of the numerous raw materials, parts and components purchased for assembly of our therapeutic and diagnostic sleep disorder products, most are off-the-shelf items available from multiple vendors. We generally manufacture to our internal sales forecasts and fill orders as received.
Business Acquisitions
Fiscal year ended June 30, 2007
Western Medical Marketing (WMM). On October 4, 2006 we acquired the business assets of WMM, a distribution business operating in the Pacific Northwest region of the U.S. for a total cash consideration of $0.3 million. The acquisition has been accounted as a purchase and accordingly the results of operations of WMM have been included in our consolidated financial statements since October 4, 2006. An amount of $0.3 million, representing the excess of the purchase price over the fair value of the identifiable net assets acquired, has been recorded as goodwill. We have completed our purchase price allocation at June 30, 2007.
Fiscal year ended June 30, 2006
PolarMed Holding AS (PolarMed). As disclosed in our consolidated financial statements and Form 10-K for the year ended June 30, 2006, we acquired 100% of the outstanding stock of
- 31 -
Table of Contents
PolarMed, the holding company for PolarMed AS and its affiliates, on December 1, 2005, for net cash consideration of $6.5 million. This was comprised of $6.8 million in consideration less $0.3 million of cash acquired. Additionally, as part of the acquisition, we assumed debt of $1.5 million. Under the purchase agreement, we may also be required to make additional future payments of up to $3.0 million based on the achievement of certain performance milestones following the acquisition through December 31, 2008. Of the $3.0 million in potential future payments included within the purchase agreement, $1.0 million was paid during the year ended June 30, 2007 as a result of the successful achievement of a performance milestone. This additional payment increased the total acquisition consideration to $7.8 million from $6.8 million and increased the amount recorded as goodwill to $5.4 million from $4.4 million.
Pulmomed Medizinisch-Technische Geräte GmbH (Pulmomed). As disclosed in our consolidated financial statements and Form 10-K for the year ended June 30, 2006, we acquired 100% of the outstanding stock of Pulmomed on July 1, 2005 for net cash consideration of $2.5 million, including acquisition costs. Additionally, as part of the acquisition, we assumed debt of $1.0 million. Under the purchase agreement, we may also be required to make additional future payments of up to $0.9 million based on the achievement of certain performance milestones following the acquisition through June 30, 2007. Of the $0.9 million in potential future payments included within the purchase agreement, $0.3 million was paid during the year ended June 30, 2007 as a result of the successful achievement of a performance milestone. This additional payment was accrued at June 30, 2006, which increased the total acquisition consideration to $2.8 million from $2.5 million and increased the amount recorded as goodwill by $0.3 million to $2.1 million.
Fiscal year ended June 30, 2005
Saime SAS (Saime). We acquired 100% of the outstanding stock of Financiere ACE SAS, the holding company for Saime and its affiliates, on May 19, 2005, for net cash consideration of $40.6 million. This consisted of $51.1 million in consideration, including acquisition costs, less $10.5 million of cash acquired. An amount of $64.8 million, representing the excess of the purchase price over the fair value of the identifiable net assets acquired, has been recorded as goodwill.
Hoefner Medizintechnick GmbH (Hoefner). We acquired 100% of the outstanding stock of Hoefner on February 14, 2005, for net cash consideration of $8.2 million. This consisted of the $10.7 million in total consideration, including acquisition costs, less $2.5 million of cash acquired. Under the purchase agreement, additional future payments of up to $0.9 million were possible based on the achievement of certain performance milestones following the acquisition through December 31, 2006. Of the $0.9 million in potential future payments, $0.6 million was paid during fiscal 2006. The remaining $0.3 million of the $0.9 million was paid during the year ended June 30, 2007 as a result of the successful achievement of a performance milestone. This additional payment increased the total acquisition consideration to $11.6 million and goodwill to $9.1 million.
Resprecare BV (Resprecare). On December 1, 2004, we acquired substantially all the assets of Resprecare BV, our Dutch distributor, for initial consideration of $5.9 million in cash, including acquisition costs. Under the purchase agreement, we potentially were also required to make up to $1.4 million of additional future payments based on the achievement of certain milestones. Of these potential additional payments, $0.6 million was paid in January 2005 and a further $0.7 million was paid in January 2006 as a result of the integration of the Dutch subsidiary of our subsidiary MAP Medizin-Technologie GmbH, or MAP, with the newly-acquired Resprecare business. An amount of $4.4 million, representing the excess of the purchase price over the fair value of identifiable net assets acquired of $2.8 million, was recorded as goodwill.
- 32 -
Table of Contents
In-Process Research and Development Charge (IPR&D)
On acquisition of Saime in May 2005, we recognized as an expense a charge of $5.3 million with respect to IPR&D programs under active development by Saime that, at date of acquisition, had not reached technological feasibility and had no alternative future use.
Stock-Based Compensation Costs
We have granted stock options to personnel, including officers and directors, under our 1995 Option Plan (the 1995 Plan), our 1997 Equity Participation Plan (the 1997 Plan) and our 2006 Incentive Award Plan, as amended (the 2006 Plan and together with the 1995 Plan and the 1997 Plan, the Plans). These options have expiration dates of seven or ten years from the date of grant and vest over three or four years. We granted these options with the exercise price equal to the market value as determined at the date of grant. We have also offered to our personnel, including officers and directors, the right to purchase shares of our common stock at a discount pursuant to our employee stock purchase plan (ESPP).
As of July 1, 2005, we adopted SFAS No.123(R) using the modified prospective method, which requires measurement of compensation expense of all stock-based awards at fair value on the date of grant and recognition of compensation expense over the service period for awards expected to vest. Under this method, the provisions of SFAS No.123(R) apply to all awards granted or modified after the date of adoption. In addition, the unrecognized expense of awards not yet vested at the date of adoption, determined under the original provisions of SFAS No.123, Accounting for Stock Based Compensation (SFAS 123), shall be recognized in net income in the periods after adoption. The fair value of stock options is determined using the Black-Scholes valuation model. Such value is recognized as expense over the service period, using the graded-attribution method for stock-based awards granted prior to July 1, 2005 and the straight-line method for stock-based awards granted after July 1, 2005.
The fair value of stock options granted under the Plans and purchase rights granted under our ESPP is estimated on the date of the grant using the Black-Scholes option-pricing model, assuming no dividends and the following assumptions:
| Years ended June 30 | ||||||
| 2007 | 2006 | 2005 | ||||
| Stock Options: |
||||||
| Weighted average grant date fair value |
$14.53 | $12.75 | $8.49 | |||
| Weighted average risk-free interest rate |
4.3-5.1% | 3.9-4.5% | 4.0% | |||
| Dividend yield |
- | - | - | |||
| Expected option life in years |
4.0-5.2 | 3.9-5.2 | 3.5-4.6 | |||
| Volatility |
26-30% | 28-30% | 31% | |||
| ESPP Purchase rights: |
||||||
| Weighted average risk-free interest rate |
4.9-5.1% | 3.2-4.9% | 2.3% | |||
| Dividend yield |
- | - | - | |||
| Expected option life |
6 months | 6 months | 6 months | |||
| Volatility |
30-41% | 29-41% | 31-38% | |||
Expected volatilities are based on a combination of historical volatilities of our stock and implied volatilities from traded options of our stock. The expected life represents the weighted average period of time that options granted are expected to be outstanding giving consideration to vesting schedules
- 33 -
Table of Contents
and our historical exercise patterns. The risk-free rate is based on the U.S. Treasury yield curve in effect at the time of grant for periods corresponding with the expected life of the option.
Tax Expense
Our income tax rate is governed by the laws of the regions in which our income is recognized. To date, a substantial portion of our income has been subject to income tax in Australia where the statutory rate was 30% in fiscal years 2007, 2006 and 2005. During fiscal years 2007, 2006 and 2005, our consolidated effective tax rate has fluctuated between approximately 32% and approximately 34%. These fluctuations have resulted from, and future effective tax rates will depend upon, numerous factors, including the amount of research and development expenditures for which a 125% Australian tax deduction is available, the level of non-deductible expenses, and other tax credits or benefits available to us under applicable tax laws.
We account for income taxes under the asset and liability method. Deferred tax assets and liabilities are recognized for the future tax consequences attributable to differences between the financial statement carrying amounts of existing assets and liabilities and their respective tax bases. Deferred tax assets and liabilities are measured using enacted tax rates expected to apply to taxable income in the years in which those temporary differences are expected to be recovered or settled. The effect on deferred tax assets and liabilities of a change in tax rates is recognized in income in the period that includes the enactment date.
Fiscal Year Ended June 30, 2007 Compared to Fiscal Year Ended June 30, 2006
Net Revenues. Net revenue increased for the year ended June 30, 2007 to $716.3 million from $607.0 million for the year ended June 30, 2006, an increase of $109.3 million or 18%. The increase in net revenue was attributable to an increase in unit sales of our flow generators, masks and accessories. Movements in international currencies against the U.S. dollar positively impacted revenues by approximately $20.5 million for the year ended June 30, 2007. Excluding the impact of favorable foreign currency movements, sales for the year ended June 30, 2007 increased by 15% compared to the year ended June 30, 2006.
Net revenue in North and Latin America increased for the year ended June 30, 2007 to $376.7 million from $321.0 million for the year ended June 30, 2006, an increase of $55.7 million or 17%. This growth has been generated by increased public and physician awareness of sleep-disordered breathing together with our continued investment in our sales force and marketing initiatives. Recent product releases, in particular the Adapt SV, Swift II and Mirage Quattro, have also contributed to our sales growth.
Net revenue in markets outside the Americas increased for the year ended June 30, 2007 to $339.6 million from $286.0 million for the years ended June 30, 2007 and 2006, respectively, an increase of 19%. International sales growth predominantly reflects growth in the overall sleep-disordered breathing market and the positive impact from movements of international currencies against the U.S. dollar. Excluding the positive impact from movements of international currencies, international sales grew by 12%.
Sales of flow generators for the year ended June 30, 2007 totaled $370.6 million, an increase of 17% compared to the year ended June 30, 2006, including increases of 19% in North and Latin America and 16% elsewhere. Sales of mask systems, motors and other accessories totaled $345.8 million, an increase of 19%, including increases of 16% in North and Latin America and 24% elsewhere, for the year ended June 30, 2007, compared to the year ended June 30, 2006. We believe these increases primarily reflect growth in the overall sleep-disordered breathing market and contributions from new products.
- 34 -
Table of Contents
Gross Profit. Gross profit increased for the year ended June 30, 2007 to $384.5 million from $376.9 million for the year ended June 30, 2006, an increase of $7.6 million or 2%. Gross profit as a percentage of net revenue decreased for the year ended June 30, 2007 to 54% from 62% for the year ended June 30, 2006. The decrease in gross margin is primarily due to $59.7 million of voluntary product recall expenses that we recognized during the year ended June 30 2007. Excluding voluntary product recall expenses, gross profit as a percentage of revenue was 62% for the year ended June 30, 2007, which is consistent with the year ended June 30, 2006. Stock-based compensation expenses of $1.1 million have been included in cost of sales for the year ended June 30, 2007 compared to $0.9 million for the year ended June 30, 2006.
Voluntary Product Recall Expenses. On April 23, 2007, we initiated a worldwide voluntary product recall of approximately 300,000 units of our early production S8 flow generators. In these particular units, which were manufactured between July 2004 and May 15, 2006, there is a remote potential for a short circuit in the power supply connector. Furthermore, in seven cases worldwide, device failures have led to thermal damage to the device, with a remote potential to ignite material external to the device. We are working with our distribution partners globally to provide a replacement device to patients who have an affected S8 flow generator.
The estimated cost of this recall action is $59.7 million which has been recognized as a charge to cost of sales in the condensed consolidated statement of income during the year ended June 30, 2007. At June 30, 2007, we have incurred costs of approximately $16.3 million associated with the product recall. We expect the product recall to continue throughout fiscal year 2008. We cannot assure that the actual costs of the product recall will not differ from the amount we have estimated and recognized in our financial statements.
Selling, General and Administrative Expenses. Selling, general and administrative expenses increased for the year ended June 30, 2007 to $237.3 million from $200.2 million for the year ended June 30, 2006, an increase of $37.1 million or 19%. As a percentage of net revenue, selling, general and administrative expenses for the year ended June 30, 2007 was 33% and is consistent with the year ended June 30, 2006. Stock-based compensation expenses of $14.5 million have been included within selling, general and administrative expenses for the year ended June 30, 2007 compared to $12.4 million for the year ended June 30, 2006.
The increase in selling, general and administrative expenses was primarily due to an increase in the number of sales and administrative personnel to support our growth, continued infrastructure investment, particularly in our European businesses, stock-based compensation costs and other expenses related to the increase in our sales. The increase in selling, general and administrative expenses was also attributable to net appreciation of international currencies against the U.S. dollar, which added approximately $9.0 million to our expenses for the year ended June 30, 2007, as reported in U.S. dollars. As a percentage of net revenue, we expect our future selling, general and administrative expense to continue in the historical range of 32% to 34%.
Research and Development Expenses. Research and development expenses increased for the year ended June 30, 2007 to $50.1 million from $37.2 million for the year ended June 30, 2006, an increase of $12.9 million or 35%. As a percentage of net revenue, research and development expenses were 7% for the year ended June 30, 2007 compared to 6% for the year ended June 30, 2006. Stock-based compensation costs of $2.0 million have been included within research and development expenses for both the year ended June 30, 2007 and the year ended June 30, 2006.
The increase in research and development expenses was primarily due to an increase in the number of research and development personnel, increased charges for consulting fees and an increase in technical assessments incurred to facilitate development of new products. The increase in research
- 35 -
Table of Contents
and development expenses was also attributable to net appreciation of international currencies against the U.S. dollar, which added approximately $2.4 million to our expenses for the year ended June 30, 2007, as reported in U.S. dollars. As a percentage of net revenue, we expect our future research and development expense to continue in the range of 6% to 7%.
Donations to Foundations. In the years ended June 30, 2007 and 2006, we donated $Nil and $0.8 million, respectively, to the ResMed Foundation in the United States, and the ResMed Foundation in Australia. The Foundations overall mission includes the education of both the public and physicians about the inherent dangers of untreated SDB/OSA, particularly as it relates to cerebrovascular and cardiovascular disease.
Amortization of Acquired Intangible Assets. Amortization of acquired intangible assets for the year ended June 30, 2007 totaled $6.9 million compared to $6.3 million for the year ended June 30, 2006. The increase in amortization expense is mainly attributable to the appreciation of the Euro against the U.S. dollar as the majority of the acquired intangible assets are denominated in Euros. The amortized amounts in 2007 related to acquired intangible assets associated with the acquisitions of Pulmomed, PolarMed, Saime, Hoefner and Resprecare.
Restructuring Expenses. Restructuring expenses incurred for the year ended June 30, 2007 were $Nil compared to $1.1 million for the year ended June 30, 2006. Restructuring expenses for 2006 consisted of restructure charges associated with our integration of the separate operations of ResMed Germany and MAP into a single operating unit. We have completed the relocation of our ResMed Germany operation, previously located in Moenchengladbach, to Munich and associated integration of the back office functions including customer service, logistics and administration.
Other Income (Expense), Net. Other income, net for the year ended June 30, 2007 was $7.8 million, an increase of $5.7 million over the year ended June 30, 2006. This was predominantly due to higher interest income on additional cash balances, lower interest expense due to the reduction in our convertible debt, which was converted into equity during the quarter ended March 31, 2006 and higher foreign currency gains on foreign currency transactions and hedging.
Income Taxes. Our effective income tax rate decreased to approximately 32.3% for the year ended June 30, 2007 from approximately 33.9% for the year ended June 30, 2006. Our effective income tax rate was impacted by the tax benefit associated with the voluntary product recall expense that was recognized during the year ended June 30, 2007. Excluding the impact of voluntary product recall expenses, the effective income tax rate was 31.4% for the year ended June 30, 2007.
The decrease in our effective tax rate from June 30, 2006 is primarily due to the one-time additional income tax expense of $3.5 million, which we incurred during the year ended June 30, 2006, associated with the repatriation of $75 million in dividends received from certain controlled foreign corporations. These dividend payments were made to take advantage of a temporary tax incentive under the American Jobs Creation Act of 2004, which provides an 85% exclusion from U.S. taxable income for qualifying dividends.
We continue to benefit from the Australian corporate tax rate of 30% and certain Australian research and development tax benefits because we generate a majority of our taxable income in Australia. Excluding the impact of tax expense associated with the dividend payment in fiscal year 2006, our effective tax rate was 31.2%, which is broadly consistent with our effective tax rate for fiscal year 2007.
Net Income. As a result of the factors above, our net income for the year ended June 30, 2007 was $66.3 million or $0.85 per diluted share compared to net income of $88.2 million or $1.16 per diluted
- 36 -
Table of Contents
share for the year ended June 30, 2006. The net after tax impact of the voluntary product recall expense of $41.8 million described above resulted in a reduction of diluted earnings per share of $0.53 on an after-tax basis for the year ended June 30, 2007. Excluding the impact of the voluntary product recall expense, diluted earnings per share was $1.38, an increase of 19% over the year ended June 30, 2006.
Fiscal Year Ended June 30, 2006 Compared to Fiscal Year Ended June 30, 2005
Net Revenues. Net revenue increased for the year ended June 30, 2006 to $607.0 million from $425.5 million for the year ended June 30, 2005, an increase of $181.5 million or 43%. The increase in net revenue was attributable to an increase in unit sales of our flow generators, masks and accessories and incremental sales contributed from acquisitions. Sales were negatively impacted by the appreciation of international currencies against the U.S. dollar (decreasing sales by approximately $11.3 million).
Excluding the impact of acquisitions and unfavorable foreign currency movements sales for the year ended June 30, 2006 increased by 32% compared to the year ended June 30, 2005. Net revenue in North and Latin America increased for the year ended June 30, 2006 to $321.0 million from $218.1 million for the year ended June 30, 2005, an increase of $102.9 million or 47%. This growth has been generated by increased public and physician awareness of sleep-disordered breathing together with our continued investment in our sales force and marketing initiatives. Recent product releases, in particular our Mirage Swift mask and S8 flow generator platform, have also contributed strongly to our sales growth.
Net revenue in markets outside the Americas increased for the year ended June 30, 2006 to $286.0 million from $207.4 million for the years ended June 30, 2006 and 2005 respectively, an increase of 38%. International sales growth for the year ended June 30, 2006 reflects organic growth in the overall sleep-disordered breathing market and the recent acquisitions of Resprecare, Hoefner, Saime, PolarMed and Pulmomed. These acquisitions contributed incremental revenue of $52.7 million for the year ended June 30, 2006. Excluding the impact of acquisitions and unfavourable foreign currency movements, international sales for the year ended June 30, 2006 grew by 17% compared to the year ended June 30, 2005.
Sales of flow generators for the year ended June 30, 2006 totaled $316.6 million, an increase of 51% compared to the year ended June 30, 2005, including increases of 47% in North and Latin America and 53% elsewhere. Sales of mask systems, motors and other accessories totaled $290.4 million, an increase of 35%, including increases of 47% in North and Latin America and 16% elsewhere, for the year ended June 30, 2006, compared to the year ended June 30, 2005. These increases primarily reflect growth in the overall sleep-disordered breathing market, acquisitions during the year, and new product releases, particularly the Mirage Swift Mask and our new flow generator platform, the S8.
Gross Profit. Gross profit increased for the year ended June 30, 2006 to $376.9 million from $274.9 million for the year ended June 30, 2005, an increase of $102.0 million or 37%. Gross profit as a percentage of net revenue decreased for the year ended June 30, 2006 to 62% from 65% for the year ended June 30, 2005. The reduction in gross margin reflects the change in product and geographical mix of sales with a higher proportion of sales in flow generators, which generate lower margins relative to our mask sales, and higher North and Latin American sales, which also typically generate lower margins relative to our international sales, as well as the additional stock-based compensation costs. Stock-based compensation expenses of $0.9 million have been included within cost of sales for the year ended June 30, 2006 as compared to no stock-based compensation expense for the year ended June 30, 2005.
- 37 -
Table of Contents
Selling, General and Administrative Expenses. Selling, general and administrative expenses increased for the year ended June 30, 2006 to $200.2 million from $135.7 million for the year ended June 30, 2005, an increase of $64.5 million or 48%. As a percentage of net revenue, selling, general and administrative expenses for the year ended June 30, 2006 was 33%, marginally higher than 32% in the year ended June 30, 2005. Stock-based compensation expenses of $12.4 million have been included within selling, general and administrative expenses for the year ended June 30, 2006. Excluding the impact of stock-based compensation expenses, as a percentage of net revenue, selling, general and administrative expenses for the year ended June 30, 2006 were 31%, which is marginally lower than 32% in the year ended June 30, 2005.
The increase in selling, general and administrative expenses was primarily due to stock-based compensation costs, an increase in the number of sales and administrative personnel to support our growth, the acquisitions of Resprecare, Hoefner, Saime, PolarMed and Pulmomed, continued infrastructure investment, particularly in our European businesses, and other expenses related to the increase in our sales. As a percentage of net revenue, we expect our future selling, general and administrative expense to continue in the historical range of 31% to 34%.
Research and Development Expenses. Research and development expenses increased for the year ended June 30, 2006 to $37.2 million from $30.0 million for the year ended June 30, 2005, an increase of $7.2 million or 24%. As a percentage of net revenue, research and development expenses were 6% for the year ended June 30, 2006 compared to 7% for the year ended June 30, 2005. Stock-based compensation costs of $2.0 million have been included within research and development expenses for the year ended June 30, 2006. As a percentage of net revenue, we expect our future research and development expense to continue in the range of 5% to 7%.
Donations to Foundations. In the years ended June 30, 2006 and 2005, we donated $0.8 million and $0.5 million, respectively, to the ResMed Foundation in the United States, and the ResMed Foundation in Australia. The Foundations overall mission includes the education of both the public and physicians about the inherent dangers of untreated SDB/OSA, particularly as it relates to cerebrovascular and cardiovascular disease.
In-process Research and Development Charge. No in-process research and development charge was incurred for the year ended June 30, 2006. For the year ended June 30, 2005, purchased in-process research and development of $5.3 million was expensed upon the acquisition of Saime as technological feasibility of the products under development had not been established and no further alternative uses existed.
Amortization of Acquired Intangible Assets. Amortization of acquired intangible assets for the year ended June 30, 2006 totaled $6.3 million compared to $0.9 million for the year ended June 30, 2005. The amortized amounts in 2006 related to acquired intangible assets associated with the acquisitions of Pulmomed, PolarMed, Saime, Hoefner and Resprecare.
Restructuring Expenses. Restructuring expenses incurred for the year ended June 30, 2006 were $1.1 million compared to $5.2 million for the year ended June 30, 2005. Restructuring expenses for 2006 consisted of restructure charges associated with our integration of the separate operations of ResMed Germany and MAP into a single operating unit. We have completed the relocation of our ResMed Germany operation, previously located in Moenchengladbach, to Munich and associated integration of the back office functions including customer service, logistics and administration. We plan to continue to monitor the progress of this restructure and adjust our business strategies and personnel accordingly in an effort to maximize efficiencies and cost savings.
Other Income (Expense), Net. Other income, net for the year ended June 30, 2006 was $2.1 million, an increase of $2.8 million from other expense, net of $0.7 million for the year ended
- 38 -
Table of Contents
June 30, 2005. This was predominantly due to higher interest income on additional cash balances and the lower interest expense due to the reduction in our convertible debt, which was converted into equity during the quarter ended March 31, 2006. Other factors included higher foreign currency gains on foreign currency transactions and hedging offset by an impairment loss of $1.2 million on one of our cost method investments.
Income Taxes. Our effective income tax rate increased to approximately 33.9% for the year ended June 30, 2006 from approximately 33.0% for the year ended June 30, 2005. This was primarily due to the one-time additional income tax expense of $3.5 million associated with the repatriation of $75 million in dividends received from certain controlled foreign corporations. These dividend payments were made to take advantage of a temporary tax incentive under the American Jobs Creation Act of 2004, which provides an 85% exclusion from U.S. taxable income for qualifying dividends. The repatriation of these funds to the United States provides us with increased flexibility in the utilization of cash to further our strategic objectives.
Excluding the impact of the one-time additional income tax expense of $3.5 million relating to the dividend repatriation, the effective tax rate for the year ended June 30, 2006 was 31.2%. This compares to an adjusted effective tax rate of approximately 31.2% for the year ended June 30, 2005 when excluding the impact of the non-deductible in-process research and development charge of $5.3 million incurred in the prior year. We continue to benefit from the Australian corporate tax rate of 30% and certain Australian research and development tax benefits because we generate a majority of our taxable income in Australia.
Net Income. As a result of the factors above, our net income for the year ended June 30, 2006 was $88.2 million or $1.16 per diluted share compared to net income of $64.8 million or $0.91 per diluted share for the year ended June 30, 2005. The net after tax impact of stock-based compensation costs, restructuring expenses, in-process research and development charge, amortization of acquired intangible assets and the repatriation of funds described above resulted in a reduction of diluted earnings per share of $0.26 and $0.12 on an after-tax basis, respectively, for the years ended June 30, 2006 and 2005.
Liquidity and Capital Resources
As of June 30, 2007 and June 30, 2006, we had cash and cash equivalents and marketable securities available-for-sale of $277.7 million and $219.5 million, respectively. Working capital was $466.4 million and $381.3 million at June 30, 2007 and June 30, 2006, respectively. The increase in working capital predominantly reflects the growth and profitability of the business during the year.
Inventories at June 30, 2007 increased by $41.0 million or 35% to $157.2 million compared to June 30, 2006 inventories of $116.2 million. The increase in inventories was higher than the increase of 18% in revenues in the year ended June 30, 2007 compared to the year ended June 30, 2006, which we believe reflects increased inventory levels to accommodate our increasing sales and the launch of several new products including the VPAP Malibu and Tango flow generators, and the Mirage Quattro and Mirage Liberty masks.
Accounts receivable at June 30, 2007 were $167.8 million, an increase of $29.7 million or 21% over the June 30, 2006 accounts receivable balance of $138.1 million. The increase was higher than the 18% incremental increase in revenues for the year ended June 30, 2007 compared to the year ended June 30, 2006. Accounts receivable days sales outstanding of 77 days at June 30, 2007 increased by 7 days compared to 70 days at June 30, 2006. The increase was predominantly attributable to increases in credit terms in response to competitor actions. Our allowance for doubtful accounts as a percentage of total accounts receivable at June 30, 2007 and 2006 was 2.7% and 3.3%, respectively. The credit quality of our customers remains consistent with our past experience.
- 39 -
Table of Contents
During the year ended June 30, 2007, we generated cash of $91.1 million from operations. This was lower than the cash generated from operations for the year ended June 30, 2006 of $99.0 million and was primarily the result of the decrease in net income, higher working capital balances and product recall costs. The cash generated from operations included a reduction of $12.4 million and $9.8 million for the years ended June 30, 2007 and 2006, respectively, due to the adoption of SFAS 123(R) as tax benefits associated with employee stock options exercised during the year are required to be included within cashflows from financing activities.
Capital expenditures for the years ended June 30, 2007 and 2006 aggregated $77.6 million and $102.7 million, respectively. The capital expenditures for the year ended June 30, 2007 primarily reflected the construction of our new manufacturing, research and development building, office facilities, computer hardware and software, rental and loan equipment and purchase of production tooling equipment and machinery. As a result of these capital expenditures, our balance sheet reflects net property, plant and equipment of approximately $310.6 million at June 30, 2007 compared to $245.4 million at June 30, 2006.
During the year ended June 30, 2007, we completed the construction of our new research and development and office facilities at our existing site in Sydney, Australia. We incurred construction costs of $12 million to complete our new building for the year ended June 30, 2007. We also commenced an extension to our manufacturing facility in Sydney, Australia. We have incurred $7 million during the year and estimate additional construction cost of approximately $7 million to complete the project. We expect to complete this extension within the next fiscal year and to fund the project through a combination of cash on hand and cash generated from operations.
On July 7, 2005, we purchased a 9.78-acre parcel of land in San Diego for $21.0 million. The new location at Kearney Mesa, San Diego will allow us to develop a new corporate headquarters. We commenced construction of our new corporate headquarters during 2007 and to date have incurred expenditures of $4 million. We estimate additional construction costs of $91 million to complete the project. We expect to complete the project in March 2009 and to fund the project through a combination of cash on hand and our undrawn revolving loan of $75 million.
Details of contractual obligations at June 30, 2007 are as follows:
| Payments Due by Period | |||||||||||||||||||||
| In $000s | Total | 2008 | 2009 | 2010 | 2011 | 2012 | Thereafter | ||||||||||||||
| Long-Term Debt | $ | 115,434 | $ | 28,272 | $ | 43,885 | $ | 16,933 | $ | 20,319 | $ | 6,025 | $ | - | |||||||
| Operating Leases | 34,506 | 9,634 | 8,188 | 6,198 | 3,900 | 2,227 | 4,359 | ||||||||||||||
| Capital Leases | 564 | 78 | 78 | 78 | 78 | 78 | 174 | ||||||||||||||
| Unconditional Purchase Obligations | 33,763 | 31,969 | 876 | 876 | 21 | 21 | - | ||||||||||||||
| Total Contractual Cash Obligations | $ | 184,267 | $ | 69,953 | $ | 53,027 | $ | 24,085 | $ | 24,318 | $ | 8,351 | $ | 4,533 | |||||||
- 40 -
Table of Contents
Details of other commercial commitments at June 30, 2007 are as follows:
| In $000s | Total Amounts Committed |
Amount of Commitment Expiration Per Period | |||||||||||||||||||
| 2008 | 2009 | 2010 | 2011 | 2012 | Thereafter | ||||||||||||||||
| Standby Letters of Credit | $ | 36 | $ | 36 | $ | - | $ | - | $ | - | $ | - | $ | - | |||||||
| Guarantees* | 57,426 | 607 | 25 | 270 | 54,627 | - | 1,897 | ||||||||||||||
| Total Commercial Commitments | $ | 57,462 | $ | 643 | $ | 25 | $ | 270 | $ | 54,627 | $ | - | $ | 1,897 | |||||||
*The above guarantees mainly relate to security provided as part of our Syndicated Facility Agreement and requirements under contractual obligations with insurance companies transacting with our German subsidiaries.
During fiscal year 2006, and pursuant to the Indenture dated June 20, 2001 between us and American Stock Transfer & Trust Company, as trustee, holders of all of the 4% Convertible Subordinated Notes (the Notes) due 2006 converted the Notes into an aggregate of 3,737,593 shares of our common stock, par value $0.004. The Notes were converted into 33 shares of our common stock for each $1,000 principal amount of the Notes, at a conversion price of $30.30 per share. The dilutive impact of these conversions has been reflected in our reported earnings per share.
On March 13, 2006, our wholly-owned subsidiaries ResMed Corp., ResMed Motor Technologies Inc. and ResMed EAP Holdings Inc. entered into a Second Amended and Restated Revolving Loan Agreement with Union Bank of California, N.A. as administrative agent for the Lenders (the Loan Agreement), that provides for a revolving loan of up to $75 million. The Loan Agreement also contains customary covenants, including certain financial covenants and an obligation that we maintain certain financial ratios, including a maximum ratio of total debt to EBITDA (as defined in the Loan Agreement), a fixed charge coverage ratio, a minimum tangible net worth, and that certain of our subsidiaries maintain a minimum EBITDA and liquidity. We are currently in compliance with all of these covenants. Draws under the revolving loans must be made before March 1, 2011, at which time all unpaid principal and interest under both loans must be repaid. The outstanding principal amount due under the loans will bear interest at a rate equal to LIBOR plus 0.75% to 1.00% (depending on the applicable leverage ratio). At June 30, 2007 there were no amounts outstanding under the Loan Agreement.
On June 8, 2006, our wholly-owned Australian subsidiary, ResMed Limited, entered into a Syndicated Facility Agreement with HSBC Bank Australia Limited as original financier, facility agent and security trustee, that provides for a loan in three tranches.
Tranche A is a EUR 50 million term loan facility that refinances all amounts outstanding under a previous syndicated facility agreement dated May 16, 2005 between ResMed Limited and HSBC Bank Australia Limited, to fund the obligations of our wholly-owned French subsidiary ResMed SA under its agreement to acquire Saime. Tranche A bears interest at a rate equal to LIBOR for deposits denominated in EUR plus a margin of 0.80% or 0.90%, depending on the ratio of the total debt to EBITDA of ResMed Inc. and its subsidiaries, which we refer to as the ResMed Group, for the most recently completed fiscal year for the applicable interest period. Payments of principal must be made to reduce the total outstanding principal amount of Tranche A to EUR 37.75 million on June 30, 2008, EUR 27.5 million on June 30, 2009, EUR 15 million on December 31, 2009, and the entire outstanding principal amount must be repaid in full on June 8, 2011. At June 30, 2007, the Tranche A facility loan had an amount outstanding of $65.3 million.
Tranche B is a USD 15 million term loan facility that may only be used for the purpose of financing capital expenditures and other asset acquisitions by the ResMed Group. Tranche B bears interest at a
- 41 -
Table of Contents
rate equal to LIBOR for deposits denominated in EUR, Australian dollars, USD, or Sterling plus a margin of 0.80% or 0.90%, depending on the ratio of the total debt to EBITDA of the ResMed Group for the most recently completed fiscal year for the applicable interest period. The entire principal amount must be repaid in full on June 8, 2011. At June 30, 2007, the Tranche B facility loan had an amount outstanding of $6.0 million.
Tranche C is a USD 60 million term loan facility that may only be used for the purpose of the payment by ResMed Limited of a dividend to ResMed Holdings Limited, which will ultimately be paid to ResMed Inc. Tranche C bears interest at a rate equal to LIBOR for deposits denominated in EUR, Australian dollars or USD plus a margin of 0.70% or 0.80%, depending on the ratio of the total debt to EBITDA of the ResMed Group for the most recently completed fiscal year for the applicable interest period. Payments of principal must be made to reduce the total outstanding principal amount of Tranche C to USD 30 million on December 31, 2007 and the entire outstanding principal amount must be repaid in full by June 8, 2009. At June 30, 2007, the Tranche C facility loan had an amount outstanding of $40.1 million.
The loans under the Syndicated Facility Agreement are secured by a pledge of one hundred percent of the shares of ResMed Inc.s subsidiary, Saime, pursuant to a Pledge Agreement. The Syndicated Facility Agreement also contains customary covenants, including certain financial covenants and an obligation that ResMed Limited maintain certain financial ratios, including a minimum debt service cover ratio, a maximum ratio of total debt to EBITDA and a minimum tangible net worth. The entire principal amount of the loan and any accrued but unpaid interest may be declared immediately due and payable in the event of the occurrence of an event of default as defined in the Syndicated Facility Agreement. Events of default include, among other items, failure to make payments when due, breaches of representations, warranties or covenants, the occurrence of certain insolvency events, the occurrence of an event or change which could have a material adverse effect on ResMed Limited and its subsidiaries, and if ResMed Inc. ceases to control ResMed Limited, ResMed Corp., ResMed SAS, ResMed GmbH & Co. KG, ResMed (UK) Limited, Take Air Medical Handels-GmbH or Saime. At June 30, 2007 we were in compliance with our debt covenants.
Simultaneous with the Syndicated Facility Agreement, ResMed Limited entered into a working capital agreement with HSBC Bank Australia Limited for revolving, letter of credit and overdraft facilities up to a total commitment of 6.5 million Australian dollars for one year, and ResMed (UK) Limited entered into a working capital agreement with HSBC Bank plc for a revolving cash advance facility up to a total commitment of 3 million Sterling for one year. At June 30, 2007 there was an amount of $4.0 million outstanding under these working capital agreements.
We expect to satisfy all of our short-term liquidity requirements through a combination of cash on hand, cash generated from operations, our $75 million undrawn revolving line of credit with Union Bank of California and our $9.0 million undrawn facilities with HSBC.
The results of our international operations are affected by changes in exchange rates between currencies. Changes in exchange rates may negatively affect our consolidated net revenue and gross profit margins from international operations. We are exposed to the risk that the dollar value equivalent of anticipated cash flows would be adversely affected by changes in foreign currency exchange rates. We manage this risk through foreign currency option contracts.
Critical Accounting Principles and Estimates
The preparation of financial statements in conformity with accounting principles generally accepted in the United States of America requires us to make estimates and judgments that affect our reported amounts of assets and liabilities, revenues and expenses and related disclosures of contingent assets
- 42 -
Table of Contents
and liabilities. On an ongoing basis we evaluate our estimates, including those related to allowance for doubtful accounts, inventory reserves, warranty obligations, goodwill, impaired assets, intangible assets, income taxes, deferred tax valuation allowances, contingencies and stock-based compensation costs.
We state these accounting policies in the notes to the financial statements and at relevant sections in this discussion and analysis. The estimates are based on the information that is currently available to us and on various other assumptions that we believe to be reasonable under the circumstances. Actual results could vary from those estimates under different assumptions or conditions.
We believe that the following critical accounting policies affect the more significant judgments and estimates used in the preparation of our consolidated financial statements:
(1) Allowance for Doubtful Accounts. We maintain an allowance for doubtful accounts for estimated losses resulting from the inability of our customers to make required payments, which results in bad debt expense. We determine the adequacy of this allowance by continually evaluating individual customer receivables, considering a customers financial condition, credit history and current economic conditions. If the financial condition of our customers were to deteriorate, resulting in an impairment of their ability to make payments, additional allowances may be required.
(2) Inventory Adjustments. Inventories are stated at lower of cost or market and are determined by the first-in, first-out method. We review the components of inventory on a regular basis for excess, obsolete and impaired inventory based on estimated future usage and sales. The likelihood of any material inventory write-downs is dependent on changes in competitive conditions, new product introductions by us or our competitors, or rapid changes in customer demand.
(3) Valuation of Goodwill, Intangible and Other Long-Lived Assets. We use assumptions in establishing the carrying value, fair value and estimated lives of our goodwill, intangibles and other long-lived assets. The criteria used for these evaluations include managements estimate of the assets continuing ability to generate positive income from operations and positive cash flow in future periods compared to the carrying value of the asset, as well as the strategic significance of any identifiable intangible asset in our business objectives. If assets are considered to be impaired, the impairment recognized is the amount by which the carrying value of the assets exceeds the fair value of the assets. Useful lives and related amortization or depreciation expense are based on our estimate of the period that the assets will generate revenues or otherwise be used by us. Factors that would influence the likelihood of a material change in our reported results include significant changes in the assets ability to generate positive cash flow, loss of legal ownership or title to the asset, a significant decline in the economic and competitive environment on which the asset depends, significant changes in our strategic business objectives, utilization of the asset, and a significant change in the economic and/or political conditions in certain countries.
(4) Valuation of Deferred Income Taxes. Valuation allowances are established, when necessary, to reduce deferred tax assets to the amount expected to be realized. The likelihood of a material change in our expected realization of these assets is dependent on future taxable income, our ability to deduct tax loss carryforwards against future taxable income, the effectiveness of our tax planning and strategies among the various tax jurisdictions that we operate in, and any significant changes in the tax treatment received on our business combinations.
(5) Provision for Warranty. We provide for the estimated cost of product warranties at the time the related revenue is recognized. The amount of this provision is determined by using a financial model, which takes into consideration actual, historical expenses and potential risks associated with our different products. This financial model is then used to calculate the future probable expenses related
- 43 -
Table of Contents
to warranty and the required level of the warranty provision. Although we engage in product improvement programs and processes, our warranty obligation is affected by product failure rates and costs incurred to correct those product failures. Should actual product failure rates or estimated costs to repair those product failures differ from our estimates, revisions to our estimated warranty provision would be required.
(6) Revenue Recognition. Revenue on product sales is recorded at the time of shipment, at which time title transfers to the customer. Revenue on product sales which require customer acceptance is not recorded until acceptance is received. Royalty revenue from license agreements is recorded when earned. Service revenue received in advance from service contracts is initially deferred and recognized ratably over the life of the service contract. Revenue received in advance from rental unit contracts is initially deferred and recognized ratably over the life of the rental contract. Revenue from sale of marketing and distribution rights is initially deferred and recognized ratably as revenue over the life of the contract. Freight charges billed to customers are included in revenue. All freight-related expenses are charged to cost of sales.
We do not recognize revenues to the extent that we offer a right of return or other recourse with respect to the sale of our products or similarly offer variable sale prices for subsequent events or activities. However, as part of our sales processes we may provide upfront discounts for large orders, one time special pricing to support new product introductions, sales rebates for centralized purchasing entities or price-breaks for regular order volumes. The costs of all such programs are recorded as an adjustment to revenue. In our domestic sales activities we use a number of Manufacturer representatives to sell our products. These representatives are paid a direct commission on sales and act as an integral component of our domestic sales force. We do not sell our products to these representatives, and do not recognize revenue on such shipments. Our products are predominantly therapy-based equipment and require no installation. As such, we have no significant installation obligations.
(7) Stock-Based Compensation. In accordance with SFAS No.123(R), we measure the compensation of all stock-based awards at fair value on date of grant. Such value is recognized as compensation expense over the service period, net of estimated forfeitures. The estimation of stock awards that will ultimately vest requires judgment, and to the extent actual results differ from our estimates, such amounts will be recorded as a cumulative adjustment in the period estimates are revised. We consider many factors when estimating expected forfeitures, including the type of awards, employee class, and historical experience. Actual results may differ substantially from these estimates.
(8) Voluntary Product Recall Expenses. We recognized an accrual for the estimated cost of the voluntary product recall at the time the liability was probable and the related expenses could be reasonably estimated. The amount of this accrual was determined taking into consideration the future probable expenses directly related to the product recall including expected return rates for the affected units, unit replacement costs, legal, consulting, logistical and administrative expenses. Should actual product recall costs differ from our estimated costs or should we receive additional feedback from our ongoing discussions with regulatory bodies, revisions to our estimated product recall accrual may be required.
Recently Issued Accounting Pronouncements
In June 2006, the FASB issued FIN No. 48, Accounting for Uncertainty in Income Taxes an interpretation of FASB Statement No. 109 and subsequently in May 2007 issued FSP FIN 48-1, Definition of Settlement in FASB Interpretation No. 48 (FIN 48), which clarifies the accounting for uncertainty in income taxes recognized in the financial statements in accordance with FASB
- 44 -
Table of Contents
Statement No. 109, Accounting for Income Taxes. FIN 48 prescribes a recognition threshold and measurement attribute for the financial statement recognition and measurement of a tax position taken, or expected to be taken in a tax return. FIN 48 requires recognition of tax benefits that satisfy a greater than 50% probability threshold and also provides guidance on de-recognition, classification, interest and penalties, accounting in interim periods, disclosure, and transition. FIN 48 is effective for us beginning July 1, 2007 and we are currently assessing the potential impact that the adoption of this Interpretation will have on our financial statements.
In September 2006, the FASB issued FASB No. 157, Fair Value Measurements (FASB 157), which defines fair value, establishes a framework for measuring fair value in generally accepted accounting principles, and expands disclosures about fair value measurements. FASB 157 is effective for financial statements issued for fiscal years beginning after November 15, 2007, and interim periods within those fiscal years. We are assessing the potential impact that the adoption of this standard will have on our financial statements.
During the year ended June 30, 2007 we adopted Staff Accounting Bulletin (SAB) No. 108, Considering the Effects of Prior Year Misstatements when Quantifying Current Year Misstatements (SAB 108). SAB 108 requires analysis of misstatements using both an income statement (rollover) approach and a balance sheet (iron curtain) approach in assessing materiality and provides for a one-time cumulative effect transition adjustment. The adoption of SAB 108 did not have a material impact on our financial statements.
In February 2007, the FASB issued SFAS No. 159, The Fair Value Option for Financial Assets and Financial Liabilities (SFAS No. 159). SFAS No. 159 gives us the irrevocable option to carry many financial assets and liabilities at fair values, with changes in fair value recognized in earnings. SFAS No. 159 is effective for us beginning July 1, 2008, although early adoption is permitted. We are currently assessing the potential impact, if any, should we elect the fair value option, that adoption of SFAS No. 159 will have on our financial statements.
| ITEM 7A | QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET AND BUSINESS RISKS |
Foreign Currency Market Risk
Our reporting currency is the U.S. dollar, although the financial statements of our non-U.S. subsidiaries are maintained in their respective local currencies. We transact business in various foreign currencies, including a number of major European currencies as well as the Australian dollar. We have significant foreign currency exposure through both our Australian manufacturing activities and international sales operations.
We have established a foreign currency hedging program using purchased currency options to hedge foreign-currency-denominated financial assets, liabilities and manufacturing expenditures. The goal of this hedging program is to economically guarantee or lock-in the exchange rates on our foreign currency exposures denominated in Euros and the Australian dollar. Under this program, increases or decreases in our foreign-currency-denominated financial assets, liabilities, and firm commitments are partially offset by gains and losses on the hedging instruments. We have determined our hedge program to be a non-effective hedge as defined under SFAS No. 133. The foreign currency derivatives portfolio is recorded in the consolidated balance sheets at fair value and included in other assets or other liabilities. All movements in the fair value of the foreign currency derivatives are recorded within other income, net on our consolidated statements of income.
- 45 -
Table of Contents
The table below provides information (in U.S. dollars) on our foreign-currency-denominated financial assets by legal entity functional currency as of June 30, 2007 (in thousands):
| Foreign Currency Financial Assets | ||||||||||||||||||||||||||||||||||||
| Australian Dollar (AUD) |
US Dollar (USD) |
Euro (EUR) |
Great Britain Pound (GBP) |
Singapore Dollar (SGD) |
New Zealand Dollar (NZD) |
Swedish Krona (SEK) |
Swiss Franc (CHF) |
Norwegian Krone (NOK) |
||||||||||||||||||||||||||||
| AUD | ||||||||||||||||||||||||||||||||||||
| Functional Currency Entities: | ||||||||||||||||||||||||||||||||||||
| Assets | $ | - | $ | 77,860 | $ | 88,337 | $ | 13,579 | $ | 569 | $ | 862 | $ | 678 | $ | 3,334 | $ | 1,459 | ||||||||||||||||||
| Liability | - | (34,413 | ) | (68,907 | ) | (6,321 | ) | (5 | ) | (75 | ) | - | (7 | ) | - | |||||||||||||||||||||
| Net Total | - | 43,447 | 19,430 | 7,258 | 564 | 787 | 678 | 3,327 | 1,459 | |||||||||||||||||||||||||||
| USD | ||||||||||||||||||||||||||||||||||||
| Functional Currency Entities: | ||||||||||||||||||||||||||||||||||||
| Assets | 58,714 | - | - | - | - | - | - | - | - | |||||||||||||||||||||||||||
| Liability | - | - | - | - | - | - | - | - | - | |||||||||||||||||||||||||||
| Net Total | 58,714 | - | - | - | - | - | - | - | - | |||||||||||||||||||||||||||
| EURO | ||||||||||||||||||||||||||||||||||||
| Functional Currency Entities: | ||||||||||||||||||||||||||||||||||||
| Assets | - | 1 | 982 | - | - | - | - | - | - | |||||||||||||||||||||||||||
| Liability | (4 | ) | (191 | ) | (21 | ) | (1,245 | ) | - | - | (20 | ) | - | - | ||||||||||||||||||||||
| Net Total | (4 | ) | (190 | ) | 961 | (1,245 | ) | - | - | (20 | ) | - | - | |||||||||||||||||||||||
| GBP | ||||||||||||||||||||||||||||||||||||
| Functional Currency Entities: | ||||||||||||||||||||||||||||||||||||
| Assets | - | 689 | 7,467 | - | - | - | - | - | - | |||||||||||||||||||||||||||
| Liability | - | - | (888 | ) | - | - | - | - | (31 | ) | (14 | ) | ||||||||||||||||||||||||
| Net Total | - | 689 | 6,579 | - | - | - | - | (31 | ) | (14 | ) | |||||||||||||||||||||||||
| CHF | ||||||||||||||||||||||||||||||||||||
| Functional Currency Entities: | ||||||||||||||||||||||||||||||||||||
| Assets | 2 | 293 | 14 | 1 | - | - | - | - | - | |||||||||||||||||||||||||||
| Liability | - | (59 | ) | (941 | ) | (651 | ) | - | - | - | - | (66 | ) | |||||||||||||||||||||||
| Net Total | 2 | 234 | (927 | ) | (650 | ) | - | - | - | - | (66 | ) | ||||||||||||||||||||||||
| NOK | ||||||||||||||||||||||||||||||||||||
| Functional Currency Entities: | ||||||||||||||||||||||||||||||||||||
| Assets | - | - | - | - | - | - | - | - | - | |||||||||||||||||||||||||||
| Liability | - | (149 | ) | (78 | ) | (15 | ) | - | - | (129 | ) | - | - | |||||||||||||||||||||||
| Net Total | - | (149 | ) | (78 | ) | (15 | ) | - | - | (129 | ) | - | - | |||||||||||||||||||||||
| SEK | ||||||||||||||||||||||||||||||||||||
| Functional Currency Entities: | ||||||||||||||||||||||||||||||||||||
| Assets | - | - | - | - | - | - | - | - | - | |||||||||||||||||||||||||||
| Liability | - | (1,269 | ) | (112 | ) | (17 | ) | - | - | - | - | (1,231 | ) | |||||||||||||||||||||||
| Net Total | - | (1,269 | ) | (112 | ) | (17 | ) | - | - | - | - | (1,231 | ) | |||||||||||||||||||||||
- 46 -
Table of Contents
The table below provides information about our foreign currency derivative financial instruments and presents the information in U.S. dollar equivalents. The table summarizes information on instruments and transactions that are sensitive to foreign currency exchange rates, including foreign currency call options held at June 30, 2007. The table presents the notional amounts and weighted average exchange rates by contractual maturity dates for our foreign currency derivative financial instruments. These notional amounts generally are used to calculate payments to be exchanged under the options contracts.
| (In thousands except exchange rates) |
Fair Value Assets / (Liabilities) |
|||||||||
| As of June 30 | ||||||||||
| FY 2008 | FY 2009 | Total | 2007 | 2006 | ||||||
| Foreign Exchange Call Options | ||||||||||
| (Receive AUD$/Pay U.S.$) | ||||||||||
| Option amount | $72,000 | $45,000 | $117,000 | $3,558 | $1,035 | |||||
| Average contractual exchange rate | AUD $1 = USD 0.8088 | AUD $1 = USD 0.8383 | AUD $1 = USD 0.8199 | |||||||
| (Receive AUD$/Pay GBP$) | ||||||||||
| Option amount | $6,026 | $- | $6,026 | $82 | $- | |||||
| Average contractual exchange rate | AUD $1 = GBP 0.4300 | AUD $1 = GBP 0.4300 | ||||||||
| (Receive AUD$/Pay Euro) | ||||||||||
| Option amount | $12,191 | $4,064 | $16,255 | $209 | $144 | |||||
| Average contractual exchange rate | AUD $1 = Euro 0.6424 | AUD $1 = Euro 0.670 | AUD $1 = Euro 0.6490 | |||||||
Interest Rate Risk
We are exposed to risk associated with changes in interest rates affecting the return on our cash and cash equivalents and debt. At June 30, 2007 we had total long-term debt, including the current portion of those obligations, of $116.0 million. All of this debt is subject to variable interest rates. A hypothetical 10% change in interest rates during the year ended June 30, 2007, would not have a material impact on pretax income. We have no interest rate hedging agreements.
| ITEM 8 | CONSOLIDATED FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA |
| a) | Index to Consolidated Financial Statements |
- 47 -
Table of Contents
| b) | Supplementary Data |
Quarterly Financial Information (unaudited)The quarterly results for the years ended June 30, 2007 and 2006 are summarized below (in thousands, except per share amounts):
| 2007 | First Quarter |
Second Quarter |
Third Quarter |
Fourth Quarter |
Fiscal Year |
|||||||||||
| Net revenues |
$ | 163,605 | $ | 178,428 | $ | 182,990 | $ | 191,309 | $ | 716,332 | ||||||
| Gross profit |
101,296 | 111,758 | 54,232 | 117,206 | 384,492 | |||||||||||
| Net income/(loss) |
24,999 | 28,995 | (15,365 | ) | 27,674 | 66,302 | ||||||||||
| Basic earnings per share |
$ | 0.33 | $ | 0.38 | ($ | 0.20 | ) | $ | 0.36 | $ | 0.86 | |||||
| Diluted earnings per share |
$ | 0.32 | $ | 0.37 | ($ | 0.20 | ) | $ | 0.35 | $ | 0.85 | |||||
| 2006 | First Quarter |
Second Quarter |
Third Quarter |
Fourth Quarter |
Fiscal Year |
|||||||||||
| Net revenues |
$ | 127,127 | $ | 146,416 | $ | 162,281 | $ | 171,172 | $ | 606,996 | ||||||
| Gross profit |
80,119 | 91,726 | 100,866 | 104,184 | 376,895 | |||||||||||
| Net income |
16,442 | 22,314 | 26,362 | 23,093 | 88,211 | |||||||||||
| Basic earnings per share |
$ | 0.23 | $ | 0.31 | $ | 0.36 | $ | 0.31 | $ | 1.22 | ||||||
| Diluted earnings per share |
$ | 0.23 | $ | 0.30 | $ | 0.34 | $ | 0.30 | $ | 1.16 | ||||||
Note: Per share amounts for each quarter are computed independently, and, due to the computation formula, the sum of the four quarters may not equal the year. All share and per share information has been adjusted to reflect the two-for-one stock split effected in the form of a 100% stock dividend that was declared on August 10, 2005 and distributed on September 30, 2005.
| ITEM 9 | CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE |
None.
| ITEM 9A | CONTROLS AND PROCEDURES |
We maintain disclosure controls and procedures that are designed to ensure that information required to be disclosed in our Exchange Act reports is recorded, processed, summarized and reported within the time periods specified in the Securities and Exchange Commissions rules and forms and that such information is accumulated and communicated to our management, including our Chief Executive Officer and Chief Financial Officer, as appropriate, to allow for timely decisions regarding required disclosure. In designing and evaluating the disclosure controls and procedures, management recognizes that any controls and procedures, no matter how well designed and operated, can provide only reasonable assurance of achieving the desired control objectives, and management is required to apply its judgment in evaluating the cost-benefit relationship of possible controls and procedures.
As required by SEC Rule 13a-15(b), we carried out an evaluation, under the supervision and with the participation of our management, including our Chief Executive Officer and Chief Financial Officer, of the effectiveness of the design and operation of our disclosure controls and procedures as of June 30, 2007. Based on the foregoing, our Chief Executive Officer and Chief Financial Officer concluded that our disclosure controls and procedures were effective at the reasonable assurance level.
There has been no change in our internal controls over financial reporting during our most recent fiscal quarter that has materially affected, or is reasonably likely to materially affect, our internal controls over financial reporting.
| ITEM 9B | OTHER INFORMATION |
None.
- 48 -
Table of Contents
MANAGEMENTS REPORT ON INTERNAL CONTROL OVER FINANCIAL REPORTING
The management of the Company is responsible for establishing and maintaining adequate internal control over financial reporting as defined in Rules 13a-15(f) and 15d-15(f) under the Securities Exchange Act of 1934. The Companys internal control over financial reporting is designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles in the United States of America. The Companys internal control over financial reporting includes those policies and procedures that:
| (i) | Pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the Company; |
| (ii) | Provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the Company are being made only in accordance with authorizations of management and directors of the Company; and |
| (iii) | Provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use or disposition of the Companys assets that could have a material effect on the financial statements. |
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
Management assessed the effectiveness of the Companys internal control over financial reporting as of June 30, 2007. Management based this assessment on criteria for effective internal control over financial reporting described in Internal Control Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission. Managements assessment included an evaluation of the design of ResMed Inc.s internal control over financial reporting and testing of the operational effectiveness of its internal control over financial reporting. Management reviewed the results of its assessment with the Audit Committee of our Board of Directors.
Based on our assessment and those criteria, management has concluded that the Company did maintain effective internal control over financial reporting as of June 30, 2007.
KPMG LLP, independent registered public accounting firm, who audited and reported on the consolidated financial statements of ResMed, Inc. included in this report, has issued an attestation report on managements assessment of internal control over financial reporting.
- 49 -
Table of Contents
RESMED INC. AND SUBSIDIARIES
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
The Board of Directors and Stockholders
ResMed Inc.:
We have audited managements assessment, included in the accompanying Managements Report on Internal Control Over Financial Reporting, that ResMed Inc. maintained effective internal control over financial reporting as of June 30, 2007, based on criteria established in Internal Control Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (COSO). ResMed Inc.s management is responsible for maintaining effective internal control over financial reporting and for its assessment of the effectiveness of internal control over financial reporting. Our responsibility is to express an opinion on managements assessment and an opinion on the effectiveness of the Companys internal control over financial reporting based on our audit.
We conducted our audit in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether effective internal control over financial reporting was maintained in all material respects. Our audit included obtaining an understanding of internal control over financial reporting, evaluating managements assessment, testing and evaluating the design and operating effectiveness of internal control, and performing such other procedures as we considered necessary in the circumstances. We believe that our audit provides a reasonable basis for our opinion.
A companys internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A companys internal control over financial reporting includes those policies and procedures that (1) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (2) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (3) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the companys assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
In our opinion, managements assessment that ResMed Inc. maintained effective internal control over financial reporting as of June 30, 2007, is fairly stated, in all material respects, based on criteria established in Internal Control Integrated Framework issued by COSO. Also, in our opinion, ResMed Inc. maintained, in all material respects, effective internal control over financial reporting as of June 30, 2007, based on criteria established in Internal Control Integrated Framework issued by COSO.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States), the consolidated balance sheets of ResMed Inc. and subsidiaries as of June 30, 2007 and 2006, and the related consolidated statements of income, stockholders equity and comprehensive income, and cash flows for each of the years in the three-year period ended June 30, 2007, and our report dated August 27, 2007 expressed an unqualified opinion on those consolidated financial statements.
| /s/ KPMG LLP |
| San Diego, California |
| August 27, 2007 |
- 50 -
Table of Contents
PART III
| ITEM 10 | DIRECTORS AND EXECUTIVE OFFICERS OF THE REGISTRANT |
Information required by this Item is herein incorporated by reference from our definitive Proxy Statement for our November 8, 2007, Annual Meeting of Stockholders, which will be filed with the Securities and Exchange Commission within 120 days after June 30, 2007.
The Company has filed, as exhibits to this Annual Report on Form 10-K for the year ended June 30, 2007, the certifications of its Chief Executive Officer and Chief Financial Officer required pursuant to Section 302 of the Sarbanes-Oxley Act of 2002.
On January 8, 2007, the Company submitted to the New York Stock Exchange the Annual CEO Certification required pursuant to Section 303A.12(a) of the New York Stock Exchange Listed Company Manual.
| ITEM 11 | EXECUTIVE COMPENSATION |
Information required by this Item is herein incorporated by reference from our definitive Proxy Statement for our November 8, 2007, Annual Meeting of Stockholders, which will be filed with the Securities and Exchange Commission within 120 days after June 30, 2007.
| ITEM 12 | SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS |
Information required by this Item is herein incorporated by reference from our definitive Proxy Statement for our November 8, 2007, Annual Meeting of Stockholders, which will be filed with the Securities and Exchange Commission within 120 days after June 30, 2007.
| ITEM 13 | CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS |
No material transactions.
| ITEM 14 | PRINCIPAL ACCOUNTANT FEES AND SERVICES |
Incorporated by reference to our definitive Proxy Statement for our November 8, 2007, meeting of stockholders, which will be filed with the Securities and Exchange Commission within 120 days after June 30, 2007.
PART IV
| ITEM 15 | EXHIBITS AND CONSOLIDATED FINANCIAL STATEMENT SCHEDULES |
The following documents are filed as part of this report:
| (a) | Consolidated Financial Statements and Schedule The consolidated financial statements and schedule of the Company and its consolidated subsidiaries are set forth in the Index to Consolidated Financial Statements under Item 8 of this report. | |
| (b) | Exhibit Lists | |
| 3.1 | First Restated Certificate of Incorporation of Registrant, as amended (15) | |
| 3.2 | Third Restated By-laws of Registrant(12) | |
- 51 -
Table of Contents
| 4.1 | Form of certificate evidencing shares of Common Stock(1) | |
| 4.3 | Indenture dated as of June 20, 2001, between ResMed Inc. and American Stock Transfer & Trust Company(5) | |
| 4.4 | Registration Rights Agreement dated as of June 20, 2001, by and between ResMed Inc., Merrill Lynch & Co., Merrill Lynch, Pierce, Fenner & Smith Incorporated, Deutsche Banc Alex Brown Inc., William Blair & Company, L.L.C., Macquarie Bank Limited and UBS Warburg LLC(5) | |
| 4.5 | Registration Rights Agreement dated May 14, 2002 between ResMed Inc. and Leslie Hoffman(6) | |
| 10.1 | 1995 Stock Option Plan(1) | |
| 10.2 | 1997 Equity Participation Plan(3) | |
| 10.3 | Licensing Agreement between the University of Sydney and ResMed Ltd dated May 17, 1991, as amended(1) | |
| 10.5 | Loan Agreement between the Australian Trade Commission and ResMed Ltd dated May 3, 1994(1) | |
| 10.6 | Lease for 10121 Carroll Canyon Road, San Diego CA 92131-1109, USA(4) | |
| 10.7 | Sale and Leaseback Agreements for 97 Waterloo Rd, North Ryde, Australia(5) | |
| 10.8 | Employment Agreement dated May 14, 2002, between Servo Magnetics Inc. and Leslie Hoffman(6) | |
| 10.9 | Agreement for the purchase of Lot 6001, Norwest Business Park, Baulkham Hills, Australia(6) | |
| 10.10 | 2003 Employee Stock Purchase Plan(7) | |
| 10.11 | Loan Agreement between ResMed Limited and HSBC Bank Australia Limited (11) | |
| 10.12 | Securities Sale Agreement Financiere Ace S.A.S. dated as of May 4, 2005 (11) | |
| 10.13 | First Amended and Restated Loan Agreement, dated as of November 1, 2005, by and among ResMed Corp., ResMed EAP Holdings Inc. and Union Bank of California, N.A. (8) | |
| 10.14 | Security Agreement, dated as of November 1, 2005, by and between ResMed EAP Holdings Inc. and Union Bank of California, N.A. (8) | |
| 10.15 | Continuing Guaranty, dated as of November 1, 2005, by and between ResMed Corp. and ResMed EAP Holdings Inc and Union Bank of California, N.A. (8) | |
| 10.16 | Commercial Promissory Note, dated as of November 1, 2005, made by ResMed Corp. and ResMed EAP Holdings Inc. (8) | |
| 10.17 | Commercial Promissory Note, dated as of November 1, 2005, made by ResMed Corp. and ResMed EAP Holdings Inc. (8) | |
| 10.18 | Second Amended and Restated Revolving Loan Agreement, dated as of March 13, 2006, among ResMed Corp., Motor Technologies Inc., ResMed EAP Holdings Inc. and Union Bank of California, N.A. (9) | |
| 10.19 | Syndicated Facility Agreement, dated as of June 8, 2006, by and between ResMed Limited and HSBC Bank Australia Limited (10) | |
- 52 -
Table of Contents
| 10.20 | Deed of Guarantee and Indemnity, dated as of June 8, 2006, by and among HSBC Bank Australia Limited, ResMed Limited, ResMed SAS, ResMed GmbH & Co. KG, ResMed (UK) Limited and Take Air Medical Handels-GmbH (10) | |
| 10.21 | Deed of Guarantee and Indemnity, dated as of June 8, 2006, by and among HSBC Bank Australia Limited, ResMed Inc., ResMed Corp. and ResMed Limited (10) | |
| 10.22 | Working Capital Agreement, dated as of June 8, 2006, by and among ResMed (UK) Limited and HSBC Bank plc (10) | |
| 10.23 | Working Capital Agreement, dated as of June 8, 2006, by and among ResMed Limited and HSBC Bank Australia Limited (10) | |
| 10.24 | ResMed Inc. 2006 Incentive Award Plan (16) | |
| 10.25 | Amendment No. 1 to the ResMed Inc. 2006 Incentive Award Plan (13) | |
| 10.26 | 2006 Grant agreement for Board of Directors (15) | |
| 10.27 | 2006 Grant agreement for Executive Officers (15) | |
| 10.28 | 2006 Grant agreement for Australian Executive Officers (13) | |
| 10.29 | Form of Executive Agreement (14) | |
| 21.1 | Subsidiaries of the Registrant (15) | |
| 23.1 | Independent Registered Public Accounting Firms Consent and Report on Schedule (15) | |
| 31.1 | Certification of Chief Executive Officer Pursuant to Section 302 of Sarbanes-Oxley Act of 2002 (15) | |
| 31.2 | Certification of Chief Financial Officer Pursuant to Section 302 of Sarbanes-Oxley Act of 2002 (15) | |
| 32.1 | Certification of Chief Executive Officer and Chief Financial Officer pursuant to Section 906 of the Sarbanes-Oxley Act of 2002 (15)
|
|
(1) Incorporated by reference to the Registrants Registration Statement on Form S-1 (No. 33-91094) declared effective on June 1, 1995.
(2) Incorporated by reference to the Registrants Registration Statement on Form 8-A12G filed on April 25, 1997.
(3) Incorporated by reference to the Registrants 1997 Proxy Statement.
(4) Incorporated by reference to the Registrants Report on Form 10-K dated June 30, 1998.
(5) Incorporated by reference to the Registrants Report on Form 10-K for the year ended June 30, 2001.
(6) Incorporated by reference to the Registrants Report on Form 10-K for the year ended June 30, 2002.
(7) Incorporated by reference to the Registrants 2003 Definitive Proxy Statement dated October 13, 2007.
(8) Incorporated by reference to the Registrants Form 8-K dated November 8, 2005.
(9) Incorporated by reference to the Registrants Form 8-K dated March 13, 2006.
(10) Incorporated by reference to the Registrants Form 8-K dated June 8, 2006.
(11) Incorporated by reference to the Registrants Report on Form 10-K for the year ended June 30, 2005.
(12) Incorporated by reference to the Registrants Report on Form 8-K dated February 23, 2007.
(13) Incorporated by reference to the Registrants Report on Form 10-Q for the quarter ended December 31, 2006.
(14) Incorporated by reference to the Registrants Report on Form 8-K dated July 9, 2007.
(15) Filed herewith
(16) Incorporated by reference to the Registrants Report on Form 8-K dated November 9, 2006.
- 53 -
Table of Contents
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
The Board of Directors and Stockholders
ResMed Inc.:
We have audited the accompanying consolidated balance sheets of ResMed Inc. and subsidiaries as of June 30, 2007 and 2006, and the related consolidated statements of income, stockholders equity and comprehensive income, and cash flows for each of the years in the three-year period ended June 30, 2007. In connection with our audits of the consolidated financial statements, we also have audited financial statement schedule II. These consolidated financial statements and financial statement schedule are the responsibility of the Companys management. Our responsibility is to express an opinion on these consolidated financial statements and financial statement schedule based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements. An audit also includes assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the consolidated financial statements referred to above present fairly, in all material respects, the financial position of ResMed Inc. and subsidiaries as of June 30, 2007 and 2006, and the results of their operations and their cash flows for each of the years in the three-year period ended June 30, 2007, in conformity with U.S. generally accepted accounting principles. Also in our opinion, the related financial statement schedule, when considered in relation to the basic consolidated financial statements taken as a whole, presents fairly, in all material respects, the information set forth therein.
As discussed in Note 2 to the consolidated financial statements, effective July 1, 2005, the Company adopted the provisions of Statement of Financial Accounting Standards No. 123 (revised 2004), Share-Based Payment.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States), the effectiveness of ResMed Inc.s internal control over financial reporting as of June 30, 2007, based on criteria established in Internal ControlIntegrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (COSO), and our report dated August 27, 2007, expressed an unqualified opinion on managements assessment of, and the effective operation of, internal control over financial reporting.
/s/ KPMG LLP
San Diego, California
August 27, 2007
F1
Table of Contents
Consolidated Balance Sheets
June 30, 2007 and 2006
(In thousands, except share and per share data)
| June 30, 2007 |
June 30, 2006 |
|||||||
| Assets |
||||||||
| Current assets: |
||||||||
| Cash and cash equivalents |
$ | 257,792 | $ | 219,544 | ||||
| Marketable securities available-for-sale (note 4) |
19,950 | - | ||||||
| Accounts receivable, net of allowance for doubtful accounts of $4,704 and $4,645 at June 30, 2007 and 2006, respectively |
167,821 | 138,147 | ||||||
| Inventories, net (note 5) |
157,204 | 116,194 | ||||||
| Deferred income taxes (note 14) |
42,109 | 27,071 | ||||||
| Income taxes receivable |
7,952 | - | ||||||
| Prepaid expenses and other current assets |
15,971 | 9,763 | ||||||
| Total current assets |
668,799 | 510,719 | ||||||
| Non-current assets: |
||||||||
| Property, plant and equipment, net of accumulated depreciation of $154,559 and $115,471 at June 30, 2007 and 2006, respectively (note 7) |
310,580 | 245,376 | ||||||
| Goodwill (note 8) |
206,778 | 195,612 | ||||||
| Other intangibles (note 8) |
46,575 | 48,897 | ||||||
| Deferred income taxes (note 14) |
9,206 | 5,265 | ||||||
| Other assets |
10,104 | 7,052 | ||||||
| Total non-current assets |
583,243 | 502,202 | ||||||
| Total assets |
$ | 1,252,042 | $ | 1,012,921 | ||||
| Liabilities and Stockholders Equity |
||||||||
| Current liabilities: |
||||||||
| Accounts payable |
$ | 53,039 | $ | 45,045 | ||||
| Accrued expenses (notes 9 and 20) |
98,324 | 40,901 | ||||||
| Deferred revenue |
18,865 | 15,344 | ||||||
| Income taxes payable |
3,410 | 22,841 | ||||||
| Deferred income taxes (note 14) |
415 | 435 | ||||||
| Current portion of long-term debt (note 10) |
28,350 | 4,869 | ||||||
| Total current liabilities |
202,403 | 129,435 | ||||||
| Non-current liabilities: |
||||||||
| Deferred income taxes (note 14) |
18,297 | 17,642 | ||||||
| Deferred revenue |
12,472 | 11,484 | ||||||
| Long-term debt (note 10) |
87,648 | 116,212 | ||||||
| Total non-current liabilities |
118,417 | 145,338 | ||||||
| Total liabilities |
320,820 | 274,773 | ||||||
| Commitments and contingencies (notes 17, 18 and 19) |
- | - | ||||||
| Stockholders equity: (note 12) |
||||||||
| Preferred stock, $0.01 par value, 2,000,000 shares authorized; none issued |
- | - | ||||||
| Common stock, $0.004 par value, 200,000,000 shares authorized; issued and outstanding 77,617,450 at June 30, 2007 and 75,772,316 at June 30, 2006 (excluding 2,304,918 and 2,254,918 shares held as Treasury stock respectively) |
311 | 303 | ||||||
| Additional paid-in capital |
421,701 | 353,464 | ||||||
| Retained earnings |
436,954 | 370,652 | ||||||
| Treasury stock, at cost |
(43,497 | ) | (41,405 | ) | ||||
| Accumulated other comprehensive income (note 6) |
115,753 | 55,134 | ||||||
| Total stockholders equity |
931,222 | 738,148 | ||||||
| Total liabilities and stockholders equity |
$ | 1,252,042 | $ | 1,012,921 | ||||
See accompanying notes to consolidated financial statements.
F2
Table of Contents
Consolidated Statements of Income
Years Ended June 30, 2007, 2006 and 2005
(In thousands, except per share data)
| June 30, 2007 |
June 30, 2006 |
June 30, 2005 |
||||||||
| Net revenues |
$ | 716,332 | $ | 606,996 | $ | 425,505 | ||||
| Cost of sales (A) |
272,140 | 230,101 | 150,645 | |||||||
| Voluntary product recall expenses (note 20) |
59,700 | - | - | |||||||
| Gross profit |
384,492 | 376,895 | 274,860 | |||||||
| Operating expenses: |
||||||||||
| Selling, general and administrative (A) |
237,326 | 200,168 | 135,703 | |||||||
| Research and development (A) |
50,106 | 37,216 | 30,014 | |||||||
| Donations to research foundations |
- | 760 | 500 | |||||||
| In-process research and development charge |
- | - | 5,268 | |||||||
| Amortization of acquired intangible assets |
6,897 | 6,327 | 870 | |||||||
| Restructuring expenses (note 11) |
- | 1,124 | 5,152 | |||||||
| Total operating expenses |
294,329 | 245,595 | 177,507 | |||||||
| Income from operations |
90,163 | 131,300 | 97,353 | |||||||
| Other income (expenses): |
||||||||||
| Interest income (expense), net |
6,477 | 1,320 | (808 | ) | ||||||
| Other, net (note 13) |
1,333 | 774 | 81 | |||||||
| Total other income (expenses), net |
7,810 | 2,094 | (727 | ) | ||||||
| Income before income taxes |
97,973 | 133,394 | 96,626 | |||||||
| Income taxes (note 14) |
31,671 | 45,183 | 31,841 | |||||||
| Net income |
$ | 66,302 | $ | 88,211 | $ | 64,785 | ||||
| Basic earnings per share |
$ | 0.86 | $ | 1.22 | $ | 0.94 | ||||
| Diluted earnings per share (note 2-j) |
$ | 0.85 | $ | 1.16 | $ | 0.91 | ||||
| Basic shares outstanding |
76,709 | 72,307 | 68,643 | |||||||
| Diluted shares outstanding |
78,253 | 77,162 | 74,942 | |||||||
| (A) Includes stock-based compensation costs as follows (note 2-r): |
||||||||||
| Cost of sales |
$ | 1,081 | $ | 891 | $ | - | ||||
| Selling, general and administrative |
14,474 | 12,372 | - | |||||||
| Research and development |
1,950 | 2,042 | - | |||||||
| Total stock-based compensation costs |
$ | 17,505 | $ | 15,305 | $ | - | ||||
See accompanying notes to consolidated financial statements.
F3
Table of Contents
Consolidated Statements of Stockholders Equity and Comprehensive Income
Years ended June 30, 2007, 2006 and 2005
(In thousands)
| Common Stock | Additional Paid-in Capital |
Treasury Stock | Retained Earnings |
Accumulated Other Comprehensive Income (Loss) |
Comprehensive Income |
||||||||||||||||||||||||||
| Shares | Amount | Shares | Amount | Total | |||||||||||||||||||||||||||
| Balance, June 30, 2004 |
69,589 | $ | 270 | $ | 132,740 | (1,772 | ) | $ | (30,440 | ) | $ | 217,656 | $ | 41,273 | $ | 361,499 | |||||||||||||||
| Common stock issued on exercise of options |
2,634 | 9 | 36,766 | 36,775 | |||||||||||||||||||||||||||
| Common stock issued on employee stock purchase plan |
134 | 2 | 2,649 | 2,651 | |||||||||||||||||||||||||||
| Treasury stock purchases |
(1 | ) | (482 | ) | (10,965 | ) | (10,966 | ) | |||||||||||||||||||||||
| Tax benefit from exercise of options |
7,710 | 7,710 | |||||||||||||||||||||||||||||
| Comprehensive income: |
|||||||||||||||||||||||||||||||
| Net income |
64,785 | 64,785 | $ | 64,785 | |||||||||||||||||||||||||||
| Other comprehensive income: |
|||||||||||||||||||||||||||||||
| Foreign currency translation adjustments |
11,617 | 11,617 | 11,617 | ||||||||||||||||||||||||||||
| Unrealized losses on marketable securities |
(6 | ) | (6 | ) | (6 | ) | |||||||||||||||||||||||||
| Comprehensive income/(loss) |
$ | 76,396 | |||||||||||||||||||||||||||||
| Balance, June 30, 2005 |
72,357 | $ | 280 | $ | 179,865 | (2,254 | ) | $ | (41,405 | ) | $ | 282,441 | $ | 52,884 | $ | 474,065 | |||||||||||||||
| Common stock issued on exercise of options (note 12) |
1,805 | 7 | 30,790 | 30,797 | |||||||||||||||||||||||||||
| Common stock issued on employee stock purchase plan (note 12) |
126 | 1 | 3,755 | 3,756 | |||||||||||||||||||||||||||
| Tax benefit from stock options exercised |
10,107 | 10,107 | |||||||||||||||||||||||||||||
| Common stock issued on conversion of convertible subordinated notes |
3,738 | 15 | 113,235 | 113,250 | |||||||||||||||||||||||||||
| FAS123(R) stock-based compensation costs |
15,712 | 15,712 | |||||||||||||||||||||||||||||
| Comprehensive income (note 6): |
|||||||||||||||||||||||||||||||
| Net income |
88,211 | 88,211 | $ | 88,211 | |||||||||||||||||||||||||||
| Other comprehensive income: |
|||||||||||||||||||||||||||||||
| Foreign currency translation adjustments |
2,250 | 2,250 | 2,250 | ||||||||||||||||||||||||||||
| Comprehensive income/(loss) |
$ | 90,461 | |||||||||||||||||||||||||||||
| Balance, June 30, 2006 |
78,026 | $ | 303 | $ | 353,464 | (2,254 | ) | $ | (41,405 | ) | $ | 370,652 | $ | 55,134 | $ | 738,148 | |||||||||||||||
| Common stock issued on exercise of options (note 12) |
1,747 | 7 | 32,672 | 32,679 | |||||||||||||||||||||||||||
| Common stock issued on employee stock purchase plan (note 12) |
148 | 1 | 5,388 | 5,389 | |||||||||||||||||||||||||||
| Treasury stock purchases |
(50 | ) | (2,092 | ) | (2,092 | ) | |||||||||||||||||||||||||
| Tax benefit from exercise of options |
12,682 | 12,682 | |||||||||||||||||||||||||||||
| FAS123(R) stock-based compensation costs |
17,495 | 17,495 | |||||||||||||||||||||||||||||
| Comprehensive income: |
|||||||||||||||||||||||||||||||
| Net income |
66,302 | 66,302 | 66,302 | ||||||||||||||||||||||||||||
| Other comprehensive income: |
|||||||||||||||||||||||||||||||
| Foreign currency translation adjustments |
60,619 | 60,619 | 60,619 | ||||||||||||||||||||||||||||
| Comprehensive income/(loss) |
$ | 126,921 | |||||||||||||||||||||||||||||
| Balance, June 30, 2007 |
79,921 | $ | 311 | $ | 421,701 | (2,304 | ) | $ | (43,497 | ) | $ | 436,954 | $ | 115,753 | $ | 931,222 | |||||||||||||||
See accompanying notes to consolidated financial statements.
F4
Table of Contents
Consolidated Statements of Cash Flows
Years ended June 30, 2007, 2006 and 2005
(In thousands)
| June 30, 2007 |
June 30, 2006 |
June 30, 2005 |
||||||||||
| Cash flows from operating activities: |
||||||||||||
| Net income |
$ | 66,302 | $ | 88,211 | $ | 64,785 | ||||||
| Adjustments to reconcile net income to net cash provided by operating activities: |
||||||||||||
| Voluntary product recall expenses |
59,700 | - | - | |||||||||
| Depreciation and amortization |
47,948 | 40,970 | 28,292 | |||||||||
| Provision for warranties |
1,542 | 1,890 | 501 | |||||||||
| Deferred income taxes |
(18,900 | ) | (11,915 | ) | (7,997 | ) | ||||||
| Foreign currency options revaluation |
(1,091 | ) | 3,796 | 293 | ||||||||
| Amortization of deferred borrowing costs |
193 | 649 | 834 | |||||||||
| Stock-based compensation costs |
17,505 | 15,305 | - | |||||||||
| Tax benefit from stock options exercised |
(12,398 | ) | (9,753 | ) | - | |||||||
| Impairment of cost-method investment |
- | 1,156 | - | |||||||||
| Release of profit on sale of building |
- | - | (2,371 | ) | ||||||||
| Purchased in-process research and development write-off |
- | - | 5,268 | |||||||||
| Changes in operating assets and liabilities, net of effect of acquisitions: |
||||||||||||
| Accounts receivable, net |
(25,612 | ) | (28,287 | ) | (27,996 | ) | ||||||
| Inventories, net |
(30,467 | ) | (25,041 | ) | (22,562 | ) | ||||||
| Prepaid expenses and other current assets |
(12,035 | ) | (2,432 | ) | 558 | |||||||
| Accounts payable, accrued expenses, income taxes and other liabilities |
(1,581 | ) | 24,479 | 31,474 | ||||||||
| Net cash provided by operating activities |
91,106 | 99,028 | 71,079 | |||||||||
| Cash flows from investing activities: |
||||||||||||
| Purchases of property, plant and equipment |
(77,556 | ) | (102,749 | ) | (39,691 | ) | ||||||
| Capitalized interest |
(412 | ) | (1,100 | ) | - | |||||||
| Purchases of marketable securitiesavailable for sale |
(21,950 | ) | (2,000 | ) | (401,546 | ) | ||||||
| Proceeds from sale of marketable securitiesavailable for sale |
2,000 | 2,002 | 413,576 | |||||||||
| Patent registration costs |
(3,965 | ) | (3,115 | ) | (2,819 | ) | ||||||
| Business acquisitions, net of cash acquired of $Nil ($262 in 2006 and $12,982 in 2005) |
(1,912 | ) | (10,526 | ) | (54,425 | ) | ||||||
| Purchases of non-trading investments |
(1,622 | ) | (2,386 | ) | (1,873 | ) | ||||||
| Net cash used in investing activities |
(105,417 | ) | (119,874 | ) | (86,778 | ) | ||||||
| Cash flows from financing activities: |
||||||||||||
| Proceeds from issuance of common stock, net |
38,260 | 34,389 | 39,426 | |||||||||
| Repayment of assumed borrowings from acquisitions |
- | (2,195 | ) | (65,764 | ) | |||||||
| Repayment of borrowings |
(20,060 | ) | (46,308 | ) | - | |||||||
| Proceeds from borrowings, net of borrowing costs |
9,590 | 102,128 | 62,500 | |||||||||
| Tax benefit from stock option exercises |
12,398 | 9,753 | - | |||||||||
| Purchases of treasury stock |
(2,092 | ) | - | (10,966 | ) | |||||||
| Net cash provided by financing activities |
38,096 | 97,767 | 25,196 | |||||||||
| Effect of exchange rate changes on cash |
14,463 | 438 | 3,781 | |||||||||
| Net increase in cash and cash equivalents |
38,248 | 77,359 | 13,278 | |||||||||
| Cash and cash equivalents at beginning of the year |
219,544 | 142,185 | 128,907 | |||||||||
| Cash and cash equivalents at end of the year |
$ | 257,792 | $ | 219,544 | $ | 142,185 | ||||||
| Supplemental disclosure of cash flow information: |
||||||||||||
| Income taxes paid, net of refunds |
$ | 65,643 | $ | 44,873 | $ | 24,747 | ||||||
| Interest paid, net of capitalized interest |
5,426 | 4,566 | 4,530 | |||||||||
| Fair value of assets acquired in acquisitions |
$ | - | $ | 11,517 | $ | 89,188 | ||||||
| Liabilities assumed |
- | (6,816 | ) | (99,270 | ) | |||||||
| Goodwill on acquisition |
1,588 | 5,553 | 78,949 | |||||||||
| Acquisition costs accrued |
324 | (1,279 | ) | (1,726 | ) | |||||||
| Acquisition costs paid |
- | 1,813 | 266 | |||||||||
| Cash paid for acquisition, including acquisition costs |
$ | 1,912 | $ | 10,788 | $ | 67,407 | ||||||
See accompanying notes to consolidated financial statements.
F5
Table of Contents
RESMED INC. AND SUBSIDIARIES
Notes to Consolidated Financial Statements
| (1) | Organization and Basis of Presentation |
ResMed Inc. (referred to herein as we, us, our or the Company) is a Delaware corporation formed in March 1994 as a holding company for the ResMed Group. Through our subsidiaries, we design, manufacture and market equipment for the diagnosis and treatment of sleep-disordered breathing and other respiratory disorders, including obstructive sleep apnea. Our manufacturing operations are located in Australia, Germany, France and the United States of America. Major distribution and sales sites are located in the United States of America, Germany, France, the United Kingdom, Switzerland, Australia and Sweden.
All share and per share information in the notes has been adjusted to reflect the two-for-one stock split effected in the form of a 100% stock dividend that was declared on August 10, 2005 and distributed on September 30, 2005.
| (2) | Summary of Significant Accounting Policies |
| (a) | Basis of Consolidation |
The consolidated financial statements include the accounts of the Company and its wholly owned subsidiaries. All significant inter-company transactions and balances have been eliminated in consolidation.
The preparation of financial statements in conformity with accounting principles generally accepted in the United States of America requires management estimates and assumptions that affect amounts reported in the financial statements and accompanying notes. Actual results could differ from managements estimates.
| (b) | Revenue Recognition |
Revenue on product sales is generally recorded upon shipment, at which time title transfers to the customer. Revenue on product sales which require customer acceptance is not recorded until acceptance is received. Royalty revenue from license agreements is recorded when earned. Service revenue received in advance from service contracts is initially deferred and recognized ratably over the life of the service contract. Revenue received in advance from rental unit contracts is initially deferred and recognized ratably over the life of the rental contract. Revenue from sale of marketing or distribution rights is initially deferred and recognized ratably as revenue over the life of the contract. Freight charges billed to customers are included in revenue. All shipping and handling related expenses are charged to cost of sales.
We do not recognize revenues to the extent that we offer a right of return or other recourse with respect to the sale of our products, other than returns for product defects or other warranty claims, nor do we recognize revenues if we offer variable sale prices for subsequent events or activities. However, as part of our sales processes we may provide upfront discounts for large orders, one time special pricing to support new product introductions, sales rebates for centralized purchasing entities or price-breaks for regular order volumes. The costs of all such programs are recorded as an adjustment to revenue. In our U.S. sales activities we use a number of manufacturer representatives to sell our products. These representatives are paid a direct commission on sales and act as an integral component of our U.S. sales force. We do not sell our products to these representatives and do not recognize revenue on such shipments. Our products are predominantly therapy-based equipment and require no installation. As such, we have no significant installation obligations.
F6
Table of Contents
RESMED INC. AND SUBSIDIARIES
Notes to Consolidated Financial Statements
| (2) | Summary of Significant Accounting Policies, Continued |
| (c) | Cash and Cash Equivalents |
Cash equivalents include certificates of deposit, commercial paper and other highly liquid investments and are stated at cost, which approximates market. Investments with original maturities of 90 days or less are considered to be cash equivalents for purposes of the consolidated statements of cash flows.
| (d) | Inventories |
Inventories are stated at the lower of cost, determined principally by the first-in, first-out method, or net realizable value. We review and provide for any product obsolescence in our manufacturing and distribution operations with assessments of individual products and components (based on estimated future usage and sales) being performed throughout the year.
| (e) | Property, Plant and Equipment |
Property, plant and equipment, including rental equipment, is recorded at cost. Depreciation expense is computed using the straightline method over the estimated useful lives of the assets, generally two to ten years except for buildings which are depreciated over an estimated useful life of 40 years. Maintenance and repairs are charged to expense as incurred.
We capitalize interest in connection with the construction of facilities. Actual construction costs incurred relating to facilities under active development qualify for interest capitalization. Interest capitalization ceases when the construction of a facility is complete and available for use. During the years ended June 30, 2007 and 2006, we capitalized $0.4 million and $1.1 million, respectively, of interest relating to such construction costs.
| (f) | Intangible Assets |
The registration costs for new patents are capitalized and amortized over the estimated useful life of the patent, generally five years. In the event of a patent being superseded, the unamortized costs are written off immediately.
Other intangible assets are amortized on a straight-line basis over their estimated useful lives, which range from seven to nine years. We evaluate the recoverability of intangible assets periodically and take into account events or circumstances that warrant revised estimates of useful lives or that indicate that impairment exists. All of our intangible assets are subject to amortization. No impairment of intangible assets has been identified during any of the periods presented.
| (g) | Goodwill |
We conducted our annual review for goodwill impairment during the final quarter of fiscal 2007. In conducting our review of goodwill impairment, we identified reporting units, being components of our operating segment, as each of the entities acquired and giving rise to the goodwill. The fair value for each reporting unit was determined based on estimated discounted cash flows. Our goodwill impairment review involved a two-step process as follows:
| Step 1- | Compare the fair value for each reporting unit to its carrying value, including goodwill. For each reporting unit where the carrying value, including goodwill, exceeds the reporting units fair value, move on to step 2. If a reporting units fair value exceeds the carrying value, no further work is performed and no impairment charge is necessary. |
F7
Table of Contents
RESMED INC. AND SUBSIDIARIES
Notes to Consolidated Financial Statements
| (2) | Summary of Significant Accounting Policies, Continued |
| Step 2- | Allocate the fair value of the reporting unit to its identifiable tangible and non-goodwill intangible assets and liabilities. This will derive an implied fair value for the goodwill. Then, compare the implied fair value of the reporting units goodwill with the carrying amount of the reporting units goodwill. If the carrying amount of the reporting units goodwill is greater than the implied fair value of its goodwill, an impairment loss must be recognized for the excess. |
The results of the review indicated that no impaired goodwill exists.
| (h) | Foreign Currency |
The consolidated financial statements of our nonU.S. subsidiaries, whose functional currencies are other than U.S. dollars, are translated into U.S. dollars for financial reporting purposes. Assets and liabilities of nonU.S. subsidiaries whose functional currencies are other than the U.S. dollar are translated at period end exchange rates, and revenue and expense transactions are translated at average exchange rates for the period. Cumulative translation adjustments are recognized as part of comprehensive income, as detailed in Note 6, and are included in accumulated other comprehensive income in the consolidated balance sheets until such time as the subsidiary is sold or substantially or completely liquidated. Gains and losses on transactions denominated in other than the functional currency of the entity are reflected in operations.
| (i) | Research and Development |
All research and development costs are expensed in the period incurred.
| (j) | Earnings per Share |
We calculate earnings per share in accordance with Statement of Financial Accounting Standards (SFAS) No. 128, Earnings per Share (SFAS 128), as amended by SFAS No. 123(R), Share Based Payments (SFAS 123(R)). SFAS 128 requires the presentation of basic earnings per share and diluted earnings per share. Basic earnings per share is computed by dividing the net income available to common stockholders by the weighted average number of shares of common stock outstanding. For purposes of calculating diluted earnings per share, net income is adjusted for the after-tax amount of interest associated with convertible debt, and the denominator includes both the weighted average number of shares of common stock outstanding and the number of dilutive common stock equivalents such as stock options and convertible notes.
The weighted average shares used to calculate basic earnings per share were 76,709,000, 72,307,000 and 68,643,000 for the years ended June 30, 2007, 2006 and 2005, respectively. The difference between basic earnings per share and diluted earnings per share is attributable to the impact of outstanding stock options during the periods presented and the assumed conversion of our convertible notes. Stock options had the effect of increasing the number of shares used in the calculation (by application of the treasury stock method) by 1,544,000, 2,346,000 and 2,561,000 for the years ended June 30, 2007, 2006 and 2005, respectively. The assumed conversion of our convertible notes had the effect of increasing the number of shares used in the calculation by Nil, 2,509,000 and 3,738,000 for the years ended June 30, 2007, 2006 and 2005, respectively. During the year ended June 30, 2006 all of our convertible notes were converted to common stock.
F8
Table of Contents
RESMED INC. AND SUBSIDIARIES
Notes to Consolidated Financial Statements
| (2) | Summary of Significant Accounting Policies, Continued |
Stock options of 3,164,000, 1,103,000 and 568,000 for the years ended June 30, 2007, 2006 and 2005 respectively, were not included in the computation of diluted earnings per share as the effect of exercising these options would have been anti-dilutive.
Basic and diluted earnings per share for the years ended June 30, 2007, 2006 and 2005 are calculated as follows (in thousands except per share data):
| 2007 | 2006 | 2005 | |||||||
| Numerator: |
|||||||||
| Net income |
$ | 66,302 | $ | 88,211 | $ | 64,785 | |||
| Adjustment for interest and deferred borrowing costs, net of income tax effect (1) |
- | 1,660 | 3,285 | ||||||
| Net income, used in calculating diluted earnings per share |
$ | 66,302 | $ | 89,871 | $ | 68,070 | |||
| Denominator: |
|||||||||
| Basic weighted-average common shares outstanding |
76,709 | 72,307 | 68,643 | ||||||
| Effect of dilutive securities: |
|||||||||
| Stock options |
1,544 | 2,346 | 2,561 | ||||||
| Convertible subordinated notes |
- | 2,509 | 3,738 | ||||||
| Diluted potential common shares |
1,544 | 4,855 | 6,299 | ||||||
| Diluted weighted average shares |
78,253 | 77,162 | 74,942 | ||||||
| Basic earnings per share |
$ | 0.86 | $ | 1.22 | $ | 0.94 | |||
| Diluted earnings per share(1) |
$ | 0.85 | $ | 1.16 | $ | 0.91 | |||
| (1) |
Diluted earnings per share has been calculated after adjusting the numerator (net income) by $Nil, $1,660,000 and $3,285,000 for the years ended June 30, 2007, 2006 and 2005, respectively, for the effect of assumed conversion of our convertible notes, and the related reduction in interest expense, net of tax. |
| (k) | Financial Instruments |
The carrying value of financial instruments, such as cash and cash equivalents, marketable securities available-for-sale, accounts receivable and accounts payable, approximate their fair value because of their short-term nature. Foreign currency option contracts are marked to market and therefore reflect their fair value. We do not hold or issue financial instruments for trading purposes.
The fair value of financial instruments is defined as the amount at which the instrument could be exchanged in a current transaction between willing parties.
| (l) | Foreign Exchange Risk Management |
We enter into various types of foreign exchange contracts in managing our foreign exchange risk, including derivative financial instruments encompassing forward exchange contracts and foreign currency options.
F9
Table of Contents
RESMED INC. AND SUBSIDIARIES
Notes to Consolidated Financial Statements
| (2) | Summary of Significant Accounting Policies, Continued |
The purpose of our foreign currency hedging activities is to protect us from adverse exchange rate fluctuations with respect to net cash movements resulting from the sales of products to foreign customers and Australian manufacturing activities. We enter into foreign currency option contracts to hedge anticipated sales and manufacturing costs, principally denominated in Australian dollars and Euros. The terms of such foreign currency option contracts generally do not exceed three years.
Our foreign currency derivatives portfolio represents a cash flow hedge program against the net cash flow of our international manufacturing operations. We have determined our hedge program to be a non-effective hedge as defined under SFAS 133. The foreign currency derivatives portfolio is recorded in the consolidated balance sheets at fair value and included in other assets or other liabilities.
All movements in the fair value of the foreign currency derivatives are recorded within other income, net in our consolidated statements of income.
We are exposed to credit-related losses in the event of non-performance by counter parties to financial instruments. The credit exposure of foreign exchange options at June 30, 2007 and June 30, 2006 was $3.8 million and $1.2 million, respectively, which represents the positive fair value of options held by us.
We held foreign currency option contracts with notional amounts totaling $139.3 million and $193.4 million at June 30, 2007 and 2006, respectively, to hedge foreign currency items. These contracts mature at various dates before December 2008.
| (m) | Income Taxes |
We account for income taxes under the asset and liability method. We recognize deferred tax assets and liabilities for the future tax consequences attributable to differences between the financial statement carrying amounts of existing assets and liabilities and their respective tax bases. Deferred tax assets and liabilities are measured using enacted tax rates expected to apply to taxable income in the years in which those temporary differences are expected to be recovered or settled. The effect on deferred tax assets and liabilities of a change in tax rates is recognized in income in the period that includes the enactment date.
| (n) | Marketable Securities |
Management determines the appropriate classification of our investments in debt and equity securities at the time of purchase and re-evaluates such determination at each balance sheet date. Debt securities for which we do not have the intent or ability to hold to maturity are classified as available-for-sale. Securities available-for-sale are carried at fair value, with the unrealized gains and losses, net of tax, reported in accumulated other comprehensive income.
At June 30, 2007 and 2006, the investments in debt securities were classified on the accompanying consolidated balance sheet as marketable securities-available-for-sale. These investments are diversified among high credit quality securities in accordance with our investment policy.
| (o) | Warranty |
Estimated future warranty costs related to certain products are charged to operations in the period in which the related revenue is recognized. The liability for warranty costs are included in accrued expenses in our consolidated balance sheets.
F10
Table of Contents
RESMED INC. AND SUBSIDIARIES
Notes to Consolidated Financial Statements
| (2) | Summary of Significant Accounting Policies, Continued |
Changes in the liability for product warranty for the year ended June 30, 2007 are as follows (in thousands):
| Balance at July 1, 2006 |
$ | 4,653 | ||
| Warranty accruals for the year ended June 30, 2007 |
2,755 | |||
| Warranty costs incurred for the year ended June 30, 2007 |
(1,214 | ) | ||
| Foreign currency translation adjustments |
846 | |||
| Balance at June 30, 2007 |
$ | 7,040 | ||
| (p) | Impairment of Long-Lived Assets |
We periodically evaluate the carrying value of long-lived assets to be held and used, including certain identifiable intangible assets, when events and circumstances indicate that the carrying amount of an asset may not be recovered. Recoverability of assets to be held and used is measured by a comparison of the carrying amount of an asset to future net cash flows expected to be generated by the asset. If such assets are considered to be impaired, the impairment to be recognized is measured by the amount by which the carrying amount of the assets exceeds the fair value of the assets. Assets to be disposed of are reported at the lower of the carrying amount or fair value less costs to sell.
| (q) | Cost-Method Investments |
The aggregate carrying amount of our cost-method investments at June 30, 2007 and June 30, 2006 was $4.6 million and $4.1 million, respectively. We review the carrying value of these investments at each balance sheet date. In fiscal 2007 and 2006, we recognized $Nil and $1.2 million, respectively, of impairment losses related to our cost-method investments, which include investments in privately held service companies, research companies and public companies. The expense associated with this impairment has been included in the other income (expense) line within the consolidated statements of income.
At June 30, 2007, we performed an analysis of the carrying value of these investments and an unrealized loss of $1.7 million was identified in relation to an investment in a publicly listed company. The severity of the impairment (fair value is approximately 49% less than the cost) and the duration of the impairment (less than 18 months) correlate with a devaluation in the actual share price. As the investee is a publicly listed entity its share price will fluctuate with general market movements, however because we have no intention to sell this investment, and as the investee is involved in the growing sleep-disordered breathing industry we do not consider this investment to be other-than-temporary impaired at June 30, 2007. Except for this unrealized loss, we have determined that the fair values of our other investments exceeded their carrying values.
| (r) | Stock-based Employee Compensation |
We have granted stock options to personnel, including officers and directors, under our 1995 Option Plan (the 1995 Plan), our 1997 Equity Participation Plan (the 1997 Plan) and our 2006 Incentive Award Plan, as amended (the 2006 Plan and together with the 1995 Plan and the 1997 Plan, the Plans). These options have expiration dates of seven or ten years from the date of grant and vest over
F11
Table of Contents
RESMED INC. AND SUBSIDIARIES
Notes to Consolidated Financial Statements
| (2) | Summary of Significant Accounting Policies, Continued |
three or four years. We granted these options with the exercise price equal to the market value as determined at the date of grant. We have also offered to our personnel, including officers, the right to purchase shares of our common stock at a discount pursuant to our employee stock purchase plan (ESPP).
| (r) | Stock-based Employee Compensation |
Prior to July 1, 2005 we applied APB Opinion No. 25, Accounting for Stock Issued to Employees and related Interpretations, in accounting for our equity plans. For periods prior to July 1, 2005, we complied with the disclosure only provisions of SFAS No. 123, Accounting for Stock-Based Compensation, or SFAS No. 123. Results for periods before July 1, 2005, have not been restated to reflect, and do not include the impact of SFAS No. 123(R) as all options granted under those plans had an exercise price equal to the market value of the underlying common stock on the date of grant (or within permitted discounted prices as it pertains to the ESPP). The following table illustrates the effect on net income and earnings per share for the year ended June 30, 2005, if we had applied the fair value recognition provisions of SFAS No. 123(R) for stock option grants using the fair value based method of accounting:
| In thousands, except per share data | 2005 | |||
| Net income, as reported |
$ | 64,785 | ||
| Deduct: Stock-based employee compensation expense determined under fair value based method, net of related tax effects |
(10,323 | ) | ||
| Pro forma net income |
$ | 54,462 | ||
| Adjustment for interest and deferred borrowing costs, net of related tax effects |
3,285 | |||
| Pro forma net income used in calculating diluted earnings per share |
$ | 57,747 | ||
| Earnings per share: |
||||
| Basic - as reported |
$ | 0.94 | ||
| Basic - pro forma |
$ | 0.80 | ||
| Diluted - as reported |
$ | 0.91 | ||
| Diluted - pro forma |
$ | 0.77 | ||
F12
Table of Contents
RESMED INC. AND SUBSIDIARIES
Notes to Consolidated Financial Statements
| (2) | Summary of Significant Accounting Policies, Continued |
As of July 1, 2005, we adopted SFAS No.123(R) using the modified prospective method, which requires measurement of compensation expense of all stock-based awards at fair value on the date of grant and recognition of compensation expense over the service period for awards expected to vest. Under this method, the provisions of SFAS No.123(R) apply to all awards granted or modified after the date of adoption. In addition, the unrecognized expense of awards not yet vested at the date of adoption, determined under the original provisions of SFAS No.123 shall be recognized in net income in the periods after adoption. The fair value of stock options is determined using the Black-Scholes valuation model. Such value is recognized as expense over the service period, using the graded-attribution method for stock-based awards granted prior to July 1, 2005 and the straight-line method for stock-based awards granted after July 1, 2005. The fair value of stock options granted under our stock option plans and purchase rights granted under our ESPP is estimated on the date of the grant using the Black-Scholes option-pricing model with the following assumptions:
| Years ended June 30 | |||||||||
| 2007 | 2006 | 2005 | |||||||
| Stock Options: |
|||||||||
| Weighted average grant date fair value |
$ | 14.53 | $ | 12.75 | $ | 8.49 | |||
| Weighted average risk-free interest rate |
4.3-5.1% | 3.9-4.5% | 4.0% | ||||||
| Expected option life in years |
4.0-5.2 | 3.9-5.2 | 3.5-4.6 | ||||||
| Volatility |
26-30% | 28-30% | 31% | ||||||
| ESPP Purchase rights: |
|||||||||
| Weighted average risk-free interest rate |
4.9-5.1% | 3.2-4.9% | 2.3% | ||||||
| Expected option life |
6 months | 6 months | 6 months | ||||||
| Volatility |
30-41% | 29-41% | 31-38% | ||||||
Expected volatilities are based on a combination of historical volatilities of our stock and implied volatilities from traded options of our stock. The expected life represents the weighted average period of time that options granted are expected to be outstanding giving consideration to vesting schedules and our historical exercise patterns. The risk-free rate is based on the U.S. Treasury yield curve in effect at the time of grant for periods corresponding with the expected life of the option.
| (3) | New Accounting Pronouncements |
In June 2006, the FASB issued FIN No. 48, Accounting for Uncertainty in Income Taxes an interpretation of FASB Statement No. 109 and subsequently in May 2007 issued FSP FIN 48-1, Definition of Settlement in FASB Interpretation No. 48 (FIN 48), which clarifies the accounting for uncertainty in income taxes recognized in the financial statements in accordance with FASB Statement No. 109, Accounting for Income Taxes. FIN 48 prescribes a recognition threshold and measurement attribute for the financial statement recognition and measurement of a tax position taken, or expected to be taken in a tax return. FIN 48 requires recognition of tax benefits that satisfy a greater than 50% probability threshold and also provides guidance on de-recognition, classification, interest and penalties, accounting in interim periods, disclosure, and transition. FIN 48 is effective for us beginning July 1, 2007 and we are currently assessing the potential impact that the adoption of this Interpretation will have on our financial statements.
In September 2006, the FASB issued FASB No. 157, Fair Value Measurements (FASB 157), which defines fair value, establishes a framework for measuring fair value in generally accepted accounting principles, and expands disclosures about fair value measurements. FASB 157 is effective for financial
F13
Table of Contents
RESMED INC. AND SUBSIDIARIES
Notes to Consolidated Financial Statements
| (3) | New Accounting Pronouncements, Continued |
statements issued for fiscal years beginning after November 15, 2007, and interim periods within those fiscal years. We are currently assessing the potential impact that the adoption of this standard will have on our financial statements.
During the year ended June 30, 2007 we adopted Staff Accounting Bulletin (SAB) No. 108, Considering the Effects of Prior Year Misstatements when Quantifying Current Year Misstatements (SAB 108). SAB 108 requires analysis of misstatements using both an income statement (rollover) approach and a balance sheet (iron curtain) approach in assessing materiality and provides for a one-time cumulative effect transition adjustment. The adoption of SAB 108 did not have a material impact on our financial statements.
In February 2007, the FASB issued SFAS No. 159, The Fair Value Option for Financial Assets and Financial Liabilities (SFAS No. 159). SFAS No. 159 gives us the irrevocable option to carry many financial assets and liabilities at fair values, with changes in fair value recognized in earnings. SFAS No. 159 is effective for us beginning July 1, 2008, although early adoption is permitted. We are currently assessing the potential impact, if any, should we elect the fair value option, that adoption of SFAS No. 159 will have on our financial statements.
| (4) | Marketable securities |
The estimated fair value of marketable securities available for sale as of June 30, 2007 and 2006 was $20.0 million and $Nil, respectively. At June 30, 2007 contractual maturities of all marketable securities-available-for-sale were due less than one year. Expected maturities may differ from contractual maturities because the issuers of the securities may have the right to prepay obligations without prepayment penalties
| (5) | Inventories |
Inventories, net were comprised of the following as of June 30, 2007 and 2006 (in thousands):
| 2007 | 2006 | |||||
| Raw materials |
$ | 68,911 | $ | 41,979 | ||
| Work in progress |
1,965 | 3,520 | ||||
| Finished goods |
86,328 | 70,695 | ||||
| $ | 157,204 | $ | 116,194 | |||
| (6) | Comprehensive Income |
The components of comprehensive income, net of tax, were as follows (in thousands):
| 2007 | 2006 | |||||
| Net income |
$ | 66,302 | $ | 88,211 | ||
| Foreign currency translation gains |
60,619 | 2,250 | ||||
| Comprehensive income |
$ | 126,921 | $ | 90,461 | ||
We do not provide for U.S. income taxes on foreign currency translation adjustments since we do not provide for such taxes on undistributed earnings of foreign subsidiaries.
F14
Table of Contents
RESMED INC. AND SUBSIDIARIES
Notes to Consolidated Financial Statements
| (7) | Property, Plant and Equipment |
Property, plant and equipment is comprised of the following as of June 30, 2007 and 2006 (in thousands):
| 2007 | 2006 | |||||||
| Machinery and equipment |
$ | 66,093 | $ | 51,854 | ||||
| Computer equipment |
73,114 | 52,277 | ||||||
| Furniture and fixtures |
27,865 | 21,572 | ||||||
| Vehicles |
2,985 | 2,795 | ||||||
| Clinical, demonstration and rental equipment |
52,128 | 40,615 | ||||||
| Leasehold improvements |
17,635 | 11,604 | ||||||
| Land |
61,503 | 55,946 | ||||||
| Buildings |
152,691 | 77,474 | ||||||
| Construction in progress |
11,125 | 46,710 | ||||||
| 465,139 | 360,847 | |||||||
| Accumulated depreciation and amortization |
(154,559 | ) | (115,471 | ) | ||||
| $ | 310,580 | $ | 245,376 | |||||
| (8) | Goodwill and Other Intangible Assets |
Changes in the carrying amount of goodwill for the year ended June 30, 2007, were as follows:
| (In thousands) | 2007 | ||
| Balance at July 1, 2006 |
$ | 195,612 | |
| Foreign currency translation adjustments |
9,578 | ||
| Payment of earn-out relating to PolarMed |
1,000 | ||
| Payment of earn-out relating to Hoefner |
331 | ||
| Acquisition of Western Medical Marketing |
257 | ||
| Balance at June 30, 2007 |
$ | 206,778 | |
Patents and other intangibles is comprised of the following as of June 30, 2007 and June 30, 2006:
| (In thousands) | June 30, 2007 |
June 30, 2006 |
||||||
| Developed/core product technology |
$ | 33,187 | $ | 31,336 | ||||
| Accumulated amortization |
(10,028 | ) | (4,992 | ) | ||||
| Developed/core product technology, net of accumulated amortization |
23,159 | 26,344 | ||||||
| Trade names |
1,761 | 1,663 | ||||||
| Accumulated amortization |
(531 | ) | (265 | ) | ||||
| Trade names, net of accumulated amortization |
1,230 | 1,398 | ||||||
| Customer relationships |
17,685 | 16,362 | ||||||
| Accumulated amortization |
(4,629 | ) | (2,094 | ) | ||||
| Customer relationships, net of accumulated amortization |
13,056 | 14,268 | ||||||
| Patents |
22,683 | 16,151 | ||||||
| Accumulated amortization |
(13,553 | ) | (9,264 | ) | ||||
| Patents, net of accumulated amortization |
9,130 | 6,887 | ||||||
| Patents and other intangibles, net of accumulated amortization |
$ | 46,575 | $ | 48,897 | ||||
F15
Table of Contents
RESMED INC. AND SUBSIDIARIES
Notes to Consolidated Financial Statements
| (8) | Goodwill and Other Intangible Assets, Continued |
Intangible assets consist of patents, customer relationships, trade names and developed/core product technology and are amortized over the estimated useful life of the assets, generally between five and nine years. There are no expected residual values related to these intangible assets.
In fiscal year 2005, as part of the acquisition of Saime, we recognized an intangible asset with respect to developed/core product technology. Specifically, this technology related to the design and architecture of the hardware and algorithms that formed part of Saimes ventilation products and is the subject of patents and other intellectual property protections. This technology is separable from goodwill as it is capable of being sold, transferred or licensed. This represents proprietary know-how predominantly associated with the following portfolio of products that were technologically feasible at the date of acquisition:
| (i) | Elisee Series: Combines all conventional ventilation modes and monitoring functions; and |
| (iii) | VS Series (including Serena, Ultra and Integra): A new generation of ventilators using new blower technology. |
Both of these series of products continue to generate revenue which is consistent with the original expectations. Although no assurance can be given that the underlying assumptions used to value the acquired developed/core product technology will transpire as estimated, we remain confident in the assumptions used and, as a result, the net return of the Saime acquisition.
Amortization expense related to identifiable intangible assets, including patents, for the year ended June 30, 2007 was $9.7 million. Estimated annual amortization expense for the years ending June 30, 2008 through June 30, 2012, including the effect of the Resprecare, Hoefner, Saime, Pulmomed and PolarMed acquisitions is shown below (in thousands):
| Fiscal Year | Amortization expense | ||
| 2008 |
$ | 10,088 | |
| 2009 |
9,637 | ||
| 2010 |
9,001 | ||
| 2011 |
8,363 | ||
| 2012 |
7,531 | ||
| (9) | Accrued Expenses |
Accrued expenses at June 30, 2007 and 2006 consist of the following (in thousands):
| 2007 | 2006 | |||||
| Product warranties |
$ | 7,040 | $ | 4,653 | ||
| Consulting and professional fees |
3,764 | 2,851 | ||||
| Value added taxes and other taxes due |
8,212 | 3,867 | ||||
| Employee related costs |
23,942 | 20,804 | ||||
| Accrued interest |
235 | 868 | ||||
| Marketing and promotional programs |
3,828 | 3,024 | ||||
| Restructuring |
48 | 138 | ||||
| Customer advance |
1,168 | 1,102 | ||||
| Voluntary product recall |
45,098 | - | ||||
| Other |
4,989 | 3,594 | ||||
| $ | 98,324 | $ | 40,901 | |||
Refer to Note 20 for further details on the voluntary product recall expenses.
F16
Table of Contents
RESMED INC. AND SUBSIDIARIES
Notes to Consolidated Financial Statements
| (10) | Long-term Debt |
Long-term debt at June 30, 2007 and 2006 consists of the following (in thousands):
| June 30, 2007 | June 30, 2006 | |||||
| Long-term loan |
$ | 28,272 | $ | 4,796 | ||
| Capital lease |
78 | 73 | ||||
| Current portion of long-term debt |
$ | 28,350 | $ | 4,869 | ||
| Long-term loan |
$ | 87,162 | $ | 115,644 | ||
| Capital lease |
486 | 568 | ||||
| Non-current portion of long-term debt |
$ | 87,648 | $ | 116,212 | ||
Convertible Subordinated Notes
During the year ended June 30, 2006 and pursuant to the Indenture dated June 20, 2001 between us and American Stock Transfer & Trust Company, as trustee, holders of all of the 4% Convertible Subordinated Notes (the Notes) due 2006 converted the Notes into an aggregate of 3,737,593 shares of our common stock, par value $0.004. The Notes were converted into 33 shares of our common stock for each $1,000 principal amount of the Notes, at a conversion price of $30.30 per share. No payment was made for accrued interest on the Notes surrendered for conversion and the dilutive impact of these conversions has been reflected in the reported earnings per share.
Previous to the conversion, on January 5, 2006, we had exercised our right to call for an early redemption of all of the Notes, which at that time had an outstanding balance of $113.25 million. We provided notice to the trustee and the holders of the Notes that we were to redeem the Notes on March 3, 2006 at a redemption price of approximately $1,008 per $1,000 principal amount of Notes, or 100.8% of the principal amount thereof plus accrued and unpaid interest to the redemption date. However, as noted above, holders of all of the Notes exercised their option to convert the Notes into our common stock.
Revolving Facility
On March 13, 2006, our wholly-owned subsidiaries ResMed Corp., Servo Magnetics Inc. and ResMed EAP Holdings Inc. entered into a Second Amended and Restated Revolving Loan Agreement with Union Bank of California, N.A. as administrative agent for the Lenders (the Loan Agreement), that provides for a revolving loan of up to $75 million. Draws under the revolving loan must be made before March 1, 2011, at which time all unpaid principal and interest must be repaid. The outstanding principal amount due under the loan will bear interest at a rate equal to LIBOR plus 0.75% to 1.00% (depending on the applicable leverage ratio). At June 30, 2007 there were no amounts outstanding under the Loan Agreement.
The obligations of ResMed Corp., Servo Magnetics Inc. and ResMed EAP Holdings Inc. under the Loan Agreement are secured by substantially all of the personal property of each of ResMed Corp., Servo Magnetics Inc. and ResMed EAP Holdings Inc., and are guaranteed by ResMed Inc. under an Amended and Restated Continuing Guaranty and Pledge Agreement, which guaranty is secured by a pledge of the equity interests in ResMed Corp., Servo Magnetics Inc. and ResMed EAP Holdings Inc. held by ResMed Inc. The Loan Agreement also contains customary covenants, including certain financial covenants and an obligation that ResMed Inc. maintain certain financial ratios, including a maximum ratio of total debt to EBITDA (as defined in the Loan Agreement), a fixed charge coverage ratio, a minimum tangible net worth, and a minimum ResMed Corp., Servo Magnetics Inc. and ResMed EAP Holdings Inc. EBITDA and liquidity. The
F17
Table of Contents
RESMED INC. AND SUBSIDIARIES
Notes to Consolidated Financial Statements
| (10) | Long-term Debt, Continued |
entire principal amount of the Loan and any accrued but unpaid interest may be declared immediately due and payable in the event of the occurrence of an event of default as defined in the Loan Agreement. Events of default include, among other items, failure to make payments when due, the occurrence of a material default in the performance of any covenants in the Loan Agreement or related document or a 35% or more change in control of ResMed Inc., ResMed Corp., Servo Magnetics Inc. or ResMed EAP Holdings Inc. At June 30, 2007, we were in compliance with our debt covenants.
Syndicated Facility
On June 8, 2006, our wholly-owned Australian subsidiary, ResMed Limited, entered into a Syndicated Facility Agreement with HSBC Bank Australia Limited as original financier, facility agent and security trustee, that provides for a loan in three tranches.
Tranche A is a EUR 50 million term loan facility that refinances all amounts outstanding under a previous syndicated facility agreement dated May 16, 2005 between ResMed Limited and HSBC Bank Australia Limited, to fund the obligations of our wholly owned French subsidiary, ResMed SAS, under its agreement to acquire Saime SA. Tranche A bears interest at a rate equal to LIBOR for deposits denominated in EUR plus a margin of 0.80% or 0.90%, depending on the ratio of the total debt to EBITDA of ResMed Inc. and its subsidiaries (the ResMed Group) for the most recently completed fiscal year for the applicable interest period. Payments of principal must be made to reduce the total outstanding principal amount of Tranche A to EUR 44.5 million on June 30, 2007, EUR 37.75 million on June 30, 2008, EUR 27.5 million on June 30, 2009, EUR 15 million on December 31, 2009, and the entire outstanding principal amount must be repaid in full on June 8, 2011. At June 30, 2007, the Tranche A facility loan had an amount outstanding of $65.3 million.
Tranche B is a USD 15 million term loan facility that may only be used for the purpose of financing capital expenditures and other asset acquisitions by the ResMed Group. Tranche B bears interest at a rate equal to LIBOR for deposits denominated in EUR, Australian dollars, USD or Sterling plus a margin of 0.80% or 0.90%, depending on the ratio of the total debt to EBITDA of the ResMed Group for the most recently completed fiscal year for the applicable interest period. The entire principal amount must be repaid in full on June 8, 2011. At June 30, 2007, the Tranche B facility loan had an amount outstanding of $6.0 million.
Tranche C is a USD 60 million term loan facility that may only be used for the purpose of the payment by ResMed Limited of a dividend to ResMed Holdings Limited, which will ultimately be paid to ResMed Inc. Tranche C bears interest at a rate equal to LIBOR for deposits denominated in EUR, Australian dollars or USD plus a margin of 0.70% or 0.80%, depending on the ratio of the total debt to EBITDA of the ResMed Group for the most recently completed fiscal year for the applicable interest period. Payments of principal must be made to reduce the total outstanding principal amount of Tranche C to USD 30 million on December 31, 2007 and the entire outstanding principal amount must be repaid in full by June 8, 2009. At June 30, 2007, the Tranche C facility loan had an amount outstanding of $40.1 million.
Simultaneous with the Syndicated Facility Agreement, ResMed Limited entered into a working capital agreement with HSBC Bank Australia Limited for revolving, letter of credit and overdraft facilities up to a total commitment of 6.5 million Australian dollars for one year, and ResMed (UK) Limited entered into a working capital agreement with HSBC Bank plc for a revolving cash advance facility up to a total commitment of 3 million Sterling for one year. At June 30, 2007, there was an amount of USD 4.0 million outstanding under these working capital agreements.
The loans under the Syndicated Facility Agreement are secured by a pledge of one hundred percent of the shares of ResMed Inc.s subsidiary, Saime SAS, pursuant to a Pledge Agreement. The Syndicated Facility
F18
Table of Contents
RESMED INC. AND SUBSIDIARIES
Notes to Consolidated Financial Statements
| (10) | Long-term Debt, Continued |
Agreement also contains customary covenants, including certain financial covenants and an obligation that ResMed Limited maintain certain financial ratios, including a minimum debt service cover ratio, a maximum ratio of total debt to EBITDA and a minimum tangible net worth. The entire principal amount of the loan and any accrued but unpaid interest may be declared immediately due and payable in the event of the occurrence of an event of default as defined in the Syndicated Facility Agreement. Events of default include, among other items, failure to make payments when due, breaches of representations, warranties or covenants, the occurrence of certain insolvency events, the occurrence of an event or change which could have a material adverse effect on ResMed Limited and its subsidiaries, and if ResMed Inc. ceases to control ResMed Limited, ResMed Corp., ResMed SAS, ResMed GmbH & Co. KG, ResMed (UK) Limited, Take Air Medical Handels-GmbH or Saime SAS.
The obligations of ResMed Limited under the loan are subject to two guarantee and indemnity agreements, one on behalf of ResMed Inc. and its U.S. subsidiary, ResMed Corp., and another on behalf of ResMeds international subsidiaries, ResMed SAS (other than Tranche C), ResMed GmbH & Co. KG, ResMed (UK) Limited and Take Air Medical Handels-GmbH. At June 30, 2007, we were in compliance with our debt covenants.
Capital Lease
As part of the acquisition of Saime we assumed a capital lease over land and buildings. This lease contains an option to purchase the property, for nominal consideration, at the end of the lease term in September 2014.
Details of contractual debt maturities at June 30, 2007 are as follows (in thousands):
| Payments Due by Period | ||||||||||||||||||
| Total | 2008 | 2009 | 2010 | 2011 | Thereafter | |||||||||||||
| Long-Term Debt |
$ | 115,434 | $ | 28,272 | $ | 43,885 | $ | 16,933 | $ | 20,319 | $ | 6,025 | ||||||
| Capital Leases |
564 | 78 | 78 | 78 | 78 | 252 | ||||||||||||
| Total |
$ | 115,998 | $ | 28,350 | $ | 43,963 | $ | 17,011 | $ | 20,397 | $ | 6,277 | ||||||
| (11) | Restructuring Expenses |
There were no restructuring expenses incurred during the year ended June 30, 2007 compared to $1.1 million incurred during the year ended June 30, 2006. The prior year restructuring expenses (predominantly one-time termination benefits) were associated with the integration of the separate operations of ResMed Germany and MAP into a single operating unit. We have substantially completed the relocation of our ResMed Germany operation (previously located in Moenchengladbach) to Munich and integration of the back office functions including customer service, logistics and administration.
F19
Table of Contents
RESMED INC. AND SUBSIDIARIES
Notes to Consolidated Financial Statements
| (11) | Restructuring Expenses, Continued |
Following is a summary of the restructuring liabilities related to the restructure and integration of the separate operations of ResMed Germany and MAP into a single operating unit, that were recorded during the years ended June 30, 2006 and June 30, 2007 (in thousands):
| Accrued employee costs |
Other accrued costs |
Total accrued costs |
||||||||||
| Balance at June 30, 2004 |
$ | - | $ | - | $ | - | ||||||
| Restructuring expenses |
4,673 | 479 | 5,152 | |||||||||
| Cash payments |
(4,451 | ) | (227 | ) | (4,678 | ) | ||||||
| Balance at June 30, 2005 |
$ | 222 | $ | 252 | $ | 474 | ||||||
| Restructuring expenses |
888 | 236 | 1,124 | |||||||||
| Cash payments |
(1,044 | ) | (408 | ) | (1,452 | ) | ||||||
| Foreign currency translation |
(28 | ) | 20 | (8 | ) | |||||||
| Balance at June 30, 2006 |
$ | 38 | $ | 100 | $ | 138 | ||||||
| Restructuring expenses |
- | - | - | |||||||||
| Cash payments |
(8 | ) | (87 | ) | (95 | ) | ||||||
| Foreign currency translation |
2 | 3 | 5 | |||||||||
| Balance at June 30, 2007 |
$ | 32 | $ | 16 | $ | 48 | ||||||
| (12) | Stockholders Equity |
Stock Options. We have granted stock options to personnel, including officers and directors, in accordance with the Plans. These options have expiration dates of seven or ten years from the date of grant and vest over three or four years. We have granted these options with an exercise price equal to the market value as determined at the date of grant.
At our Annual Meeting of Shareholders that was held on November 8, 2006, our shareholders approved the 2006 Plan. The 2006 Plan succeeds and replaces the 1997 Plan, which was previously adopted by the Board of Directors and then approved by the shareholders in November 1997. In connection with the adoption of the 2006 Plan, we have terminated the 1997 Plan as to any and all future awards. Options granted under the 1997 Plan, which remain outstanding, will continue to be governed by the 1997 Plan.
The maximum number of shares of our common stock authorized for issuance under the 2006 Plan is 7,800,000 shares. The number of securities remaining available for future issuance under the 2006 Plan at June 30, 2007 is 5,651,850. The number of shares of our common stock available for issuance under the 2006 Plan will be reduced by (i) two and one tenth (2.1) shares for each one share of common stock delivered in settlement of any full-value award, which is any award other than a stock option, stock appreciation right or other award for which the holder pays the intrinsic value and (ii) one share for each share of common stock delivered in settlement of all other awards. The maximum number of shares, which may be subject to awards granted under the 2006 Plan to any individual during any calendar year, may not exceed 1,000,000 shares of our common stock.
At June 30, 2007, there was $41.4 million in unrecognized compensation costs related to unvested stock options. This is expected to be recognized over a weighted average period of 2.9 years. The aggregate
F20
Table of Contents
RESMED INC. AND SUBSIDIARIES
Notes to Consolidated Financial Statements
| (12) | Stockholders Equity, Continued |
intrinsic value of the options outstanding and the options exercisable at June 30, 2007 was $82.6 million and $78.3 million, respectively. The aggregate intrinsic value of the options exercised during the years ended June 30, 2007 and June 30, 2006 was $48.3 million and $40.5 million, respectively. The total fair value of options that vested during the years ended June 30, 2007 and June 30, 2006 was $13.6 million and $17.1 million, respectively. The following table summarizes option activity during the year ended June 30, 2007:
The following table summarizes option activity:
| 2007 | Weighted Average Exercise Price |
2006 | Weighted Average Exercise Price |
2005 | Weighted Average Exercise Price |
||||||||||||||||
| Outstanding at beginning of year |
8,102,892 | $ | 24.26 | 8,301,408 | $ | 19.38 | 8,832,712 | $ | 16.27 | ||||||||||||
| Granted |
2,353,650 | 46.38 | 2,030,700 | 38.17 | 2,336,650 | 25.30 | |||||||||||||||
| Exercised |
(1,747,330 | ) | 18.70 | (1,805,648 | ) | 17.06 | (2,633,246 | ) | 13.97 | ||||||||||||
| Forfeited |
(302,729 | ) | 31.98 | (423,568 | ) | 26.09 | (234,708 | ) | 18.34 | ||||||||||||
| Outstanding at end of year |
8,406,483 | $ | 31.43 | 8,102,892 | $ | 24.26 | 8,301,408 | $ | 19.38 | ||||||||||||
| Exercise price range of granted options |
$ | 40.25-$52.58 | $ | 32.99-45.46 | $ | 21.9531.24 | |||||||||||||||
| Options exercisable at end of year |
4,001,157 | $ | 21.69 | 4,262,743 | $ | 18.03 | 3,987,754 | $ | 16.86 | ||||||||||||
The following table summarizes information about stock options outstanding at June 30, 2007.
| Range of Exercise Prices | Number Outstanding at June 30, 2007 |
Weighted Average Remaining Contractual Life In Years |
Number Exercisable at June 30, 2007 |
Weighted Average Remaining Contractual Life In Years |
||||
| $ 0 - $10 |
227,081 | 1.79 | 227,081 | 1.79 | ||||
| $11 - $20 |
1,759,476 | 5.16 | 1,716,136 | 5.12 | ||||
| $21 - $30 |
2,251,137 | 6.18 | 1,609,925 | 5.71 | ||||
| $31 - $40 |
1,897,239 | 8.27 | 418,890 | 8.14 | ||||
| $41 - $50 |
2,250,050 | 6.49 | 29,125 | 6.75 | ||||
| $51 - $60 |
21,500 | 6.59 | - | - | ||||
| 8,406,483 | 6.40 | 4,001,157 | 5.50 |
Employee Stock Purchase Plan (ESPP). The ESPP was approved by our shareholders at the Annual General Meeting in November 2003. Under the ESPP, participants are offered the right to purchase shares of our common stock at a discount during successive offering periods. Each offering period under the ESPP will be for a period of time determined by the Board of Directors Compensation Committee of no less than 3 months and no more than 27 months. The purchase price for our common stock under the ESPP will be the lower of 85% of the fair market value of our common stock on the date of grant or 85% of the fair market value of our common stock on the date of purchase. An individual participant cannot subscribe for more than $25,000 in common stock during any calendar year. On August 21, 2006, the Board of Directors
F21
Table of Contents
RESMED INC. AND SUBSIDIARIES
Notes to Consolidated Financial Statements
| (12) | Stockholders Equity, Continued |
approved a reduction in the number of shares available for grant under the ESPP to 500,000 shares, effective as of November 9, 2006, the date of the shareholder approval of the 2006 Plan. The number of securities remaining available for future issuance under the ESPP at June 30, 2007 is 420,624.
During fiscal year 2007, we issued 148,639 shares to our employees in two offerings at an average share price of $36.25. We recognized $1.6 million of stock compensation expense associated with the ESPP.
Preferred Stock. In April 1997, the Board of Directors authorized 2,000,000 shares of $0.01 par value preferred stock. No such shares were issued or outstanding at June 30, 2007.
Stock Purchase Rights. In April 1997, the Company implemented a plan to protect stockholders rights in the event of a proposed takeover of the Company. Under the plan, each share of the Companys outstanding common stock carries one right to purchase Series A Junior Participating Preferred Stock (the Right). The Right enables the holder, under certain circumstances, to purchase common stock of the Company or of the acquiring person at a substantially discounted price ten days after a person or group publicly announces it has acquired or has tendered an offer for 20% or more of the Companys outstanding common stock. The plan and its accompanying Rights expired pursuant to their terms in April 2007 and the Rights are no longer outstanding.
Common Stock. On June 6, 2002, the Board of Directors authorized the Company to repurchase up to 8.0 million shares of outstanding common stock. During fiscal years 2007 and 2006, the Company repurchased 50,000 and Nil shares at a cost of $2.1 million and $Nil, respectively. As of June 30, 2007, we have repurchased a total of 2.3 million shares at a cost of $43.5 million. Shares that are repurchased are classified as treasury stock pending future use and reduce the number of shares outstanding used in calculating earnings per share.
Convertible Subordinated Notes. During the year ended June 30, 2006, and pursuant to the Indenture dated June 20, 2001 between us and American Stock Transfer & Trust Company, as trustee, holders of all of the 4% Convertible Subordinated Notes due 2006 converted the notes into an aggregate of 3,737,593 shares of the Companys common stock, par value $0.004. The notes were converted into 33 shares of our common stock for each $1,000 principal amount of the notes, at a conversion price of $30.30 per share. The dilutive impact of these conversions has been reflected in the reported earnings per share.
| (13) | Other, net |
Other, net in the consolidated statements of income is comprised of the following at June 30, 2007, 2006 and 2005 (in thousands):
| 2007 | 2006 | 2005 | |||||||||
| Gain on foreign currency transactions and hedging |
$ | 1,203 | $ | 1,853 | $ | 36 | |||||
| Realized (loss) on sale of marketable securities |
- | - | (34 | ) | |||||||
| Impairment of cost method investment |
- | (1,156 | ) | - | |||||||
| Other |
130 | 77 | 79 | ||||||||
| $ | 1,333 | $ | 774 | $ | 81 | ||||||
F22
Table of Contents
RESMED INC. AND SUBSIDIARIES
Notes to Consolidated Financial Statements
| (14) | Income Taxes |
Income before income taxes for the years ended June 30, 2007, 2006 and 2005, was taxed under the following jurisdictions (in thousands):
| 2007 | 2006 | 2005 | ||||||||
| U.S. |
$ | 21,219 | $ | 5,472 | $ | (54 | ) | |||
| Non-U.S. |
76,754 | 127,922 | 96,680 | |||||||
| $ | 97,973 | $ | 133,394 | $ | 96,626 | |||||
The provision for income taxes is presented below (in thousands):
| 2007 | 2006 | 2005 | ||||||||||
| Current: |
||||||||||||
| Federal |
$ | 5,973 | $ | 7,507 | $ | 799 | ||||||
| State |
984 | 1,370 | 246 | |||||||||
| Non-U.S. |
43,614 | 48,221 | 38,793 | |||||||||
| 50,571 | 57,098 | 39,838 | ||||||||||
| Deferred: |
||||||||||||
| Federal |
(977 | ) | (3,353 | ) | 618 | |||||||
| State |
(225 | ) | (390 | ) | (29 | ) | ||||||
| Non-U.S. |
(17,698 | ) | (8,172 | ) | (8,586 | ) | ||||||
| (18,900 | ) | (11,915 | ) | (7,997 | ) | |||||||
| Provision for income taxes |
$ | 31,671 | $ | 45,183 | $ | 31,841 | ||||||
The provision for income taxes differs from the amount of income tax determined by applying the applicable U.S. federal income tax rate of 34% (35% for 2006 and 34% for 2005) to pretax income as a result of the following (in thousands):
| 2007 | 2006 | 2005 | ||||||||||
| Taxes computed at statutory U.S. rate |
$ | 33,311 | $ | 46,688 | $ | 32,853 | ||||||
| Increase (decrease) in income taxes resulting from: |
||||||||||||
| Effect of AJCA dividend repatriation |
- | 3,537 | - | |||||||||
| State income taxes, net of U.S. tax benefit |
982 | 939 | 165 | |||||||||
| Non-deductible expenses |
874 | 777 | 580 | |||||||||
| Research and development credit |
(4,092 | ) | (3,085 | ) | (2,743 | ) | ||||||
| Tax effect of deemed dividends |
1,438 | 1,846 | 590 | |||||||||
| Change in valuation allowance |
1,580 | 1,665 | 637 | |||||||||
| Effect of non-U.S. tax rates |
(2,425 | ) | (6,731 | ) | (3,419 | ) | ||||||
| In-process research and development write-off |
- | - | 1,791 | |||||||||
| Foreign tax credits |
(1,907 | ) | (1,204 | ) | - | |||||||
| Stock-based compensation expense |
1,692 | 2,006 | - | |||||||||
| Other |
218 | (1,255 | ) | 1,387 | ||||||||
| $ | 31,671 | $ | 45,183 | $ | 31,841 | |||||||
F23
Table of Contents
RESMED INC. AND SUBSIDIARIES
Notes to Consolidated Financial Statements
| (14) | Income Taxes, Continued |
Deferred tax assets and liabilities are classified as current or non-current according to the classification of the related asset or liability. The components of the Companys deferred tax assets and liabilities at June 30, 2007 and 2006 are as follows (in thousands):
| 2007 | 2006 | |||||||
| Deferred tax assets: |
||||||||
| Employee benefit obligations |
$ | 3,634 | $ | 3,274 | ||||
| Voluntary product recall accrual |
13,530 | - | ||||||
| Inventories |
1,587 | 1,408 | ||||||
| Provision for warranties |
1,681 | 992 | ||||||
| Provision for doubtful debts |
1,028 | 1,024 | ||||||
| Net operating loss carryforwards |
2,827 | 2,598 | ||||||
| Foreign tax credits |
10,416 | 9,626 | ||||||
| Unrealized foreign exchange losses |
- | 970 | ||||||
| Capital loss carryover |
490 | 521 | ||||||
| Intercompany profit in inventories |
23,660 | 18,611 | ||||||
| Stock-based compensation expense |
6,076 | 2,833 | ||||||
| Other |
2,126 | 2,448 | ||||||
| 67,055 | 44,305 | |||||||
| Less valuation allowance |
(12,612 | ) | (10,989 | ) | ||||
| Deferred tax assets |
$ | 54,443 | $ | 33,316 | ||||
| Deferred tax liabilities: |
||||||||
| Unrealized gain on foreign currency options |
$ | - | $ | (353 | ) | |||
| Unrealized foreign exchange gains |
(3,419 | ) | - | |||||
| Property, plant and equipment |
(1,494 | ) | (1,673 | ) | ||||
| Goodwill and other intangibles |
(15,905 | ) | (16,500 | ) | ||||
| Other |
(1,022 | ) | (531 | ) | ||||
| Deferred tax liabilities |
(21,840 | ) | (19,057 | ) | ||||
| Net deferred tax asset |
$ | 32,603 | $ | 14,259 | ||||
The net deferred tax assets and liabilities have been reported in the consolidated balance sheets at June 30, 2007 and 2006 as follows (in thousands):
| 2007 | 2006 | |||||||
| Current deferred tax asset |
$ | 42,109 | $ | 27,071 | ||||
| Non-current deferred tax asset |
9,206 | 5,265 | ||||||
| Current deferred tax liability |
(415 | ) | (435 | ) | ||||
| Non-current deferred tax liability |
(18,297 | ) | (17,642 | ) | ||||
| Net deferred tax asset |
32,603 | $ | 14,259 | |||||
As of June 30, 2007, the Company had $1,664,000, and $12,860,000 of U.S. state and non-U.S. net operating loss carryforwards, respectively, which expire in various years through 2025 or carry forward
F24
Table of Contents
RESMED INC. AND SUBSIDIARIES
Notes to Consolidated Financial Statements
| (14) | Income Taxes, Continued |
indefinitely. The Company also had foreign tax credit carryforwards of $10,416,000. The foreign tax credit carryforwards have expiration dates through 2017.
The valuation allowance at June 30, 2007, relates to a provision for uncertainty as to the utilization of foreign tax credits of $10,416,000, net operating loss carryforwards for certain non-U.S. countries of $1,671,000, capital loss items of $490,000 and other deferred tax assets of $34,000. We believe that it is more likely than not that the benefits of deferred tax assets, net of any valuation allowance, will be realized.
The Company has not provided for U.S. income and foreign withholding taxes on undistributed earnings from non-U.S. subsidiaries indefinitely invested outside the United States as of June 30, 2007. The total amount of these undistributed earnings at June 30, 2007 amounted to approximately $379 million. Should the Company repatriate foreign earnings, the Company would have to adjust the income tax provision in the period management determined that the Company would repatriate earnings.
| (15) | Employee Retirement Plans |
The Company contributes to a number of employee retirement plans for the benefit of its employees. These plans are detailed as follows:
(1) Australia - The Company contributes to defined contribution pension plans for each employee resident in Australia. All Australian employees, after serving a qualifying period, are entitled to benefits on retirement, disability or death. Employees may contribute additional funds to the plans. The Company contributes to the plans at the rate of 9% of the salaries of all Australian employees. Total Company contributions to the plans for the years ended June 30, 2007, 2006 and 2005, were $4,798,474, $3,846,000 and $2,849,000, respectively.
(2) United Kingdom - The Company contributes to a defined contribution plan for each permanent United Kingdom employee. All employees, after serving a three-month qualifying period, are entitled to benefit on retirement, disability or death. Employees may contribute additional funds to the plan. The Company contributes to the plan at the rate of 5% of the salaries of all United Kingdom employees. Total Company contributions to the plan were $242,586, $109,000 and $67,000 in fiscal 2007, 2006 and 2005, respectively.
(3) United States - The Company sponsors a defined contribution pension plan available to substantially all domestic employees. Company contributions to this plan are based on a percentage of employee contributions to a maximum of 3% of the employees salary. Total Company contributions to the plan were $759,702, $531,000 and $514,000 in fiscal 2007, 2006 and 2005, respectively.
(4) Switzerland - The Company sponsors a fixed return defined contribution fund for each permanent Swiss employee. As part of the Companys contribution to the fund, the Company guarantees a fixed 3% net return on accumulated contributions per annum. The Company contributes to the plan at variable rates that have averaged 10% of salaries over the last three years. Total Company contributions to the plan were $259,041, $182,000 and $85,000 in fiscal 2007, 2006 and 2005, respectively.
| (16) | Segment Information |
The Company operates solely in the sleep-disordered breathing sector of the respiratory medicine industry. The Company therefore believes that, given the single market focus of its operations and the inter-dependence of its products, the Company operates as a single operating segment. The Company assesses performance and allocates resources on the basis of a single operating entity.
F25
Table of Contents
RESMED INC. AND SUBSIDIARIES
Notes to Consolidated Financial Statements
| (16) | Segment Information, Continued |
Financial information by geographic area for the years ended June 30, 2007, 2006 and 2005, is summarized below (in thousands):
| U.S.A | Germany | Australia | France | Rest of World |
Total | |||||||||
| 2007 |
||||||||||||||
| Revenue from external customers |
$ | 376,699 | 107,938 | 19,846 | 75,984 | 135,865 | $ | 716,332 | ||||||
| Long lived assets |
$ | 60,224 | 17,813 | 218,537 | 8,083 | 16,027 | $ | 320,684 | ||||||
| 2006 |
||||||||||||||
| Revenue from external customers |
$ | 320,941 | 96,436 | 18,709 | 59,402 | 111,508 | $ | 606,996 | ||||||
| Long lived assets |
$ | 54,118 | 17,190 | 162,522 | 7,080 | 11,518 | $ | 252,428 | ||||||
| 2005 |
||||||||||||||
| Revenue from external customers |
$ | 210,495 | 72,824 | 14,160 | 47,537 | 80,489 | $ | 425,505 | ||||||
| Long lived assets |
$ | 32,090 | 11,615 | 130,310 | 2,544 | 6,900 | $ | 183,459 | ||||||
Net revenues from external customers are based on the location of the customer. Long-lived assets of geographic areas are those assets used in the Companys operations in each geographical area and excludes intangibles, deferred tax assets and goodwill.
| (17) | Commitments |
The Company leases buildings, motor vehicles and office equipment under operating leases. As part of the acquisition of Saime the Company assumed a capital lease for land and buildings. This lease contains an option to purchase the property, for nominal consideration, at the end of the lease term. Rental charges for operating leases are expensed as incurred. Rent expenses under operating leases for the years ended June 30, 2007, 2006 and 2005 were approximately $8.2 million, $7.5 million and $6.2 million, respectively. At June 30, 2007 the Company had the following future minimum lease payments under non-cancelable operating leases and capital leases (in thousands):
| Years | Capital Leases | Operating Leases | |||||
| 2008 |
$ | 99 | $ | 9,634 | |||
| 2009 |
96 | 8,188 | |||||
| 2010 |
93 | 6,198 | |||||
| 2011 |
90 | 3,900 | |||||
| 2012 |
87 | 2,227 | |||||
| Thereafter |
184 | 4,359 | |||||
| Total minimum lease payments |
649 | 34,506 | |||||
| Less: Interest portion |
(85 | ) | - | ||||
| Present value of net minimum lease payments |
$ | 564 | $ | 34,506 | |||
F26
Table of Contents
RESMED INC. AND SUBSIDIARIES
Notes to Consolidated Financial Statements
| (17) | Commitments, Continued |
Excluding lease commitments details of contractual obligations at June 30, 2007 are as follows (in thousands):
| Payments Due by Period | |||||||||||||||||||||
| In $000s | Total | 2008 | 2009 | 2010 | 2011 | 2012 | Thereafter | ||||||||||||||
| Long-Term Debt |
$ | 115,434 | $ | 28,272 | $ | 43,885 | $ | 16,933 | $ | 20,319 | $ | 6,025 | $ | - | |||||||
| Purchase Obligations |
33,763 | 31,969 | 876 | 876 | 21 | 21 | - | ||||||||||||||
| Total Contractual Obligations |
$ | 149,197 | $ | 60,241 | $ | 44,761 | $ | 17,809 | $ | 20,340 | $ | 6,046 | $ | - | |||||||
Details of other commercial commitments at June 30, 2007 are as follows (in thousands):
| Amount of Commitment Expiration Per Period | |||||||||||||||||||||
| Total | 2008 | 2009 | 2010 | 2011 | 2012 | Thereafter | |||||||||||||||
| Standby Letters of Credit |
$ | 36 | $ | 36 | $ | - | $ | - | $ | - | $ | - | $ | - | |||||||
| Guarantees* |
57,426 | 607 | 25 | 270 | 54,627 | - | 1,897 | ||||||||||||||
| Total Commercial Commitments |
$ | 57,462 | $ | 643 | $ | 25 | $ | 270 | $ | 54,627 | $ | - | $ | 1,897 | |||||||
* The above guarantees mainly relate to security provided as part of our Syndicated Facility Agreement and requirements under contractual obligations with insurance companies transacting with our German subsidiaries.
| (18) | Business Acquisitions |
Fiscal year ended June 30, 2007
Western Medical Marketing (WMM). On October 4, 2006 we acquired the business assets of WMM, a distribution business operating in the Pacific Northwest region of the U.S. for a total cash consideration of $0.3 million. The acquisition has been accounted as a purchase and accordingly the results of operations of WMM have been included in our consolidated financial statements since October 4, 2006. An amount of $0.3 million, representing the excess of the purchase price over the fair value of the identifiable net assets acquired, has been recorded as goodwill. We have completed our purchase price allocation at June 30, 2007.
Fiscal year ended June 30, 2006
PolarMed Holding AS (PolarMed). As disclosed in our consolidated financial statements and Form 10-K for the year ended June 30, 2006, we acquired 100% of the outstanding stock of PolarMed, the holding company for PolarMed AS and its affiliates, on December 1, 2005, for net cash consideration of $6.5 million. This was comprised of $6.8 million in consideration less $0.3 million of cash acquired. Additionally, as part of the acquisition, we assumed debt of $1.5 million. Under the purchase agreement, we may also be required to make additional future payments of up to $3.0 million based on the achievement of certain performance milestones following the acquisition through December 31, 2008. Of the $3.0 million in potential future payments included within the purchase agreement, $1.0 million was paid during the year ended June 30, 2007 as a result of the successful achievement of a performance milestone. This additional payment increased the total acquisition consideration to $7.8 million from $6.8 million and increased the amount recorded as goodwill to $5.4 million from $4.4 million.
F27
Table of Contents
RESMED INC. AND SUBSIDIARIES
Notes to Consolidated Financial Statements
| (18) | Business Acquisitions, Continued |
Pulmomed Medizinisch-Technische Geräte GmbH (Pulmomed). As disclosed in our consolidated financial statements and Form 10-K for the year ended June 30, 2006, we acquired 100% of the outstanding stock of Pulmomed on July 1, 2005 for net cash consideration of $2.5 million, including acquisition costs. Additionally, as part of the acquisition, we assumed debt of $1.0 million. Under the purchase agreement, we may also be required to make additional future payments of up to $0.9 million based on the achievement of certain performance milestones following the acquisition through June 30, 2007. Of the $0.9 million in potential future payments included within the purchase agreement, $0.3 million was paid during the year ended June 30, 2007 as a result of the successful achievement of a performance milestone. This additional payment was accrued at June 30, 2006, which increased the total acquisition consideration to $2.8 million from $2.5 million and increased the amount recorded as goodwill by $0.3 million to $2.1 million.
Fiscal year ended June 30, 2005
Saime SAS (Saime). We acquired 100% of the outstanding stock of Financiere ACE SAS, the holding company for Saime and its affiliates, on May 19, 2005, for net cash consideration of $40.6 million. This consisted of $51.1 million in consideration, including acquisition costs, less $10.5 million of cash acquired. An amount of $64.8 million, representing the excess of the purchase price over the fair value of the identifiable net assets acquired, has been recorded as goodwill.
Hoefner Medizintechnick GmbH (Hoefner). We acquired 100% of the outstanding stock of Hoefner on February 14, 2005, for net cash consideration of $8.2 million. This consisted of the $10.7 million in total consideration, including acquisition costs, less $2.5 million of cash acquired. Under the purchase agreement, additional future payments of up to $0.9 million were possibly based on the achievement of certain performance milestones following the acquisition through December 31, 2006. Of the $0.9 million in potential future payments, $0.6 million was paid during fiscal 2006. The remaining $0.3 million of the $0.9 million was paid during the year ended June 30, 2007 as a result of the successful achievement of a performance milestone. This additional payment increased the total acquisition consideration to $11.6 million and goodwill to $9.1 million.
Resprecare BV (Resprecare). On December 1, 2004, we acquired substantially all the assets of Resprecare BV, our Dutch distributor, for initial consideration of $5.9 million in cash, including acquisition costs. Under the purchase agreement, we potentially were also required to make up to $1.4 million of additional future payments based on the achievement of certain milestones. Of these potential additional payments, $0.6 million was paid in January 2005 and a further $0.7 million was paid in January 2006 as a result of the integration of the Dutch subsidiary of our subsidiary MAP with the newly-acquired Resprecare business. An amount of $4.4 million, representing the excess of the purchase price over the fair value of identifiable net assets acquired of $2.8 million, was recorded as goodwill.
| (19) | Legal Actions and Contingencies |
In the normal course of business, we are subject to routine litigation incidental to our business. While the results of this litigation cannot be predicted with certainty, we believe that their final outcome will not have a material adverse effect on our consolidated financial statements taken as a whole.
During September and October 2004, the Company began receiving tax assessment notices for the audit of one of its German subsidiaries by the German tax authorities for the years 1996 through 1998. Certain of these adjustments are being contested and appealed to the German tax authority office. We believe no
F28
Table of Contents
RESMED INC. AND SUBSIDIARIES
Notes to Consolidated Financial Statements
| (19) | Legal Actions and Contingencies, Continued |
additional provision is necessary for any tax adjustment that may result from the tax audit. However, the outcome of the audit cannot be predicted with certainty. Should any tax audit issues be resolved in a manner not consistent with managements expectations, the Company could be required to adjust its provision for income tax in the period of resolution.
On December 23, 2002, three former contractors of our subsidiary MAP Medizin-Technologie GmbH initiated proceedings in Munich 1 Regional Court (Proceedings No. 7 O 23286/02), petitioning the Court for a declaration of inventorship with respect to MAP German Patent Applications identified as No. 100 31 079 and 101 92 802.5 and European Patent Application No. EP 01 967 819.7. On March 10, 2005, the Court entered judgement in favor of the plaintiffs, finding that they should be identified as co-inventors in place of certain individual defendants. In April 2005, MAP filed an appeal of that decision. We do not expect the outcome of this litigation to have an adverse material effect on our consolidated financial statements.
In March 2006, an Australian university made a demand that ResMed pay extra royalties pursuant to a current patent license agreement. ResMed rejected the demand and have informed the university that it does not consider the claim to have merit. On 13 February 2007, the university commenced legal action in the Federal Court of Australia to pursue its claim against ResMed. ResMed is vigorously defending its position and does not expect the outcome of this claim to have an adverse material effect on ResMeds condensed consolidated financial statements.
| (20) | Voluntary Product Recall Expenses |
On April 23, 2007, we initiated a worldwide voluntary recall of approximately 300,000 units of our early production S8 flow generators used for the treatment of obstructive sleep apnea. In S8 devices manufactured between July 2004 and May 15, 2006, there is a remote potential for a short circuit in the power supply connector. We are working with our distribution partners globally to provide a replacement device to patients who have an affected S8 flow generator.
The estimated cost of this action is $59.7 million which has been recognized as a charge to cost of sales in the consolidated statement of income. As of June 30, 2007 we have a remaining liability of $45.1 million which is accrued in the consolidated balance sheet. These direct and incremental costs represent our best estimate of probable costs based on current available data and take into account factors such as expected return rates for the affected units, unit replacement costs, legal, consulting, logistical and temporary contractor expenses directly associated with the recall. Accordingly, should actual product recall costs differ from our estimated costs, material revisions to our estimated product recall accrual may be required.
Following is a summary of the liabilities related to the voluntary product recall that were recorded during the year ended June 30, 2007 (in thousands):
| Total accrued costs | ||||
| Balance at June 30, 2006 |
$ | - | ||
| Voluntary product recall expenses |
59,700 | |||
| Cash payments |
(16,272 | ) | ||
| Foreign currency translation |
1,670 | |||
| Balance at June 30, 2007 |
$ | 45,098 | ||
F29
Table of Contents
RESMED INC. AND SUBSIDIARIES
VALUATION AND QUALIFYING ACCOUNTS AND RESERVES
YEARS ENDED JUNE 30, 2007, 2006 AND 2005
(in thousands)
| Balance at Beginning of Period |
Charged to costs and expenses |
Other (deductions) |
Balance at end of period |
||||||||
| Year ended June 30, 2007 |
|||||||||||
| Applied against asset account |
|||||||||||
| Allowance for doubtful accounts |
$ | 4,645 | 1,173 | (1,114 | ) | $ | 4,704 | ||||
| Year ended June 30, 2006 |
|||||||||||
| Applied against asset account |
|||||||||||
| Allowance for doubtful accounts |
$ | 3,199 | 1,577 | (131 | ) | $ | 4,645 | ||||
| Year ended June 30, 2005 |
|||||||||||
| Applied against asset account |
|||||||||||
| Allowance for doubtful accounts |
$ | 3,197 | 611 | (609 | ) | $ | 3,199 | ||||
See accompanying report of independent registered public accounting firm.
Table of Contents
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
DATED August 24, 2007
ResMed Inc.
| /s/ PETER C. FARRELL |
| Peter C. Farrell |
| President and Chief Executive Officer |
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the Registrant and in the capacities and on the dates indicated.
| SIGNATURE | TITLE | DATE | ||
| /S/ PETER C. FARRELL Peter C. Farrell |
Chief Executive Officer, President, Chairman of the Board (Principal Executive Officer) |
August 24, 2007 | ||
| /S/ BRETT A. SANDERCOCK Brett A. Sandercock |
Chief Financial Officer (Principal Financial Officer and Principal Accounting Officer) |
August 24, 2007 | ||
| /S/ CHRISTOPHER G. ROBERTS Christopher G. Roberts |
Director |
August 24, 2007 | ||
| /S/ MICHAEL A. QUINN Michael A. Quinn |
Director |
August 24, 2007 | ||
| /S/ GARY W. PACE Gary W. Pace |
Director |
August 24, 2007 | ||
| /S/ DONAGH MCCARTHY Donagh McCarthy |
Director |
August 24, 2007 | ||
| /S/ RICHARD SULPIZIO Richard Sulpizio |
Director |
August 24, 2007 | ||
| /S/ RON TAYLOR Ron Taylor |
Director |
August 24, 2007 | ||
| /S/ JOHN P. WAREHAM John P. Wareham |
Director |
August 24, 2007 | ||
S-1
Table of Contents
RESMED INC. AND SUBSIDIARIES
EXHIBIT INDEX
| 3.1 |
First Restated Certificate of Incorporation of Registrant, as amended (15) |
| 3.2 |
Third Restated By-laws of Registrant (12) |
| 4.1 |
Form of certificate evidencing shares of Common Stock (1) |
| 4.3 |
Indenture dated as of June 20, 2001, between ResMed Inc. and American Stock Transfer & Trust Company (5) |
| 4.4 |
Registration Rights Agreement dated as of June 20, 2001, by and between ResMed Inc., Merrill Lynch & Co., Merrill Lynch, Pierce, Fenner & Smith Incorporated, Deutsche Banc Alex Brown Inc., William Blair & Company, L.L.C., Macquarie Bank Limited and UBS Warburg LLC (5) |
| 4.5 |
Registration Rights Agreement dated as of May 14, 2002 between ResMed Inc., and Mr Leslie Hoffman (6) |
| 10.1 |
1995 Stock Option Plan (1) |
| 10.2 |
1997 Equity Participation Plan (3) |
| 10.3 |
Licensing Agreement between the University of Sydney and ResMed Ltd dated May 17, 1991, as amended (1) |
| 10.5 |
Loan Agreement between the Australian Trade Commission and ResMed Limited dated May 3, 1994 (1) |
| 10.6 |
Lease for 10121 Carroll Canyon Road, San Diego CA 92131-1109, USA (4) |
| 10.7 |
Sale and Leaseback Agreements for 97 Waterloo Rd, North Ryde, Australia (5) |
| 10.8 |
Employment Agreement dated as of May 14, 2002, between Servo Magnetics Acquisition Inc., and Mr Leslie Hoffman (6) |
| 10.9 |
Agreement for the purchase of Lot 6001, Norwest Business Park, Baulkham Hills, Australia (6) |
| 10.10 |
2003 Employee Stock Purchase Plan (7) |
| 10.11 |
Loan Agreement between ResMed Limited and HSBC Bank Australia Limited (11) |
| 10.12 |
Securities Sale Agreement Financiere Ace S.A.S. dated as of May 4, 2005 (11) |
| 10.13 |
First Amended and Restated Loan Agreement, dated as of November 1, 2005, by and among ResMed Corp., ResMed EAP Holdings Inc. and Union Bank of California, N.A. (8) |
| 10.14 |
Security Agreement, dated as of November 1, 2005, by and between ResMed EAP Holdings Inc. and Union Bank of California, N.A. (8) |
| 10.15 |
Continuing Guaranty, dated as of November 1, 2005, by and between ResMed Corp. and ResMed EAP Holdings Inc and Union Bank of California, N.A. (8) |
| 10.16 |
Commercial Promissory Note, dated as of November 1, 2005, made by ResMed Corp. and ResMed EAP Holdings Inc. (8) |
| 10.17 |
Commercial Promissory Note, dated as of November 1, 2005, made by ResMed Corp. and ResMed EAP Holdings Inc. (8) |
| 10.18 |
Second Amended and Restated Revolving Loan Agreement, dated as of March 13, 2006, among ResMed Corp., ResMed Motor Technologies Inc., ResMed EAP Holdings Inc. and Union Bank of California, N.A. (9) |
| 10.19 |
Syndicated Facility Agreement, dated as of June 8, 2006, by and between ResMed Limited and HSBC Bank Australia Limited (10) |
| 10.20 |
Deed of Guarantee and Indemnity, dated as of June 8, 2006, by and among HSBC Bank Australia Limited, ResMed Limited, ResMed SAS, ResMed GmbH & Co. KG, ResMed (UK) Limited and Take Air Medical Handels-GmbH (10) |
Table of Contents
| 10.21 |
Deed of Guarantee and Indemnity, dated as of June 8, 2006, by and among HSBC Bank Australia Limited, ResMed Inc., ResMed Corp. and ResMed Limited (10) |
| 10.22 |
Working Capital Agreement, dated as of June 8, 2006, by and among ResMed (UK) Limited and HSBC Bank plc (10) |
| 10.23 |
Working Capital Agreement, dated as of June 8, 2006, by and among ResMed Limited and HSBC Bank Australia Limited (10) |
| 10.24 |
ResMed Inc. 2006 Incentive Award Plan (16) |
| 10.25 |
Amendment No. 1 to the ResMed Inc. 2006 Incentive Award Plan (13) |
| 10.26 |
2006 Grant agreement for Board of Directors (15) |
| 10.27 |
2006 Grant agreement for Executive Officers (15) |
| 10.28 |
2006 Grant agreement for Australian Executive Officers (13) |
| 10.29 |
Form of Executive Agreement (14) |
| 21.1 |
Subsidiaries of the Registrant (15) |
| 23.1 |
Independent Registered Public Accounting Firms Consent and Report on Schedule (15) |
| 31.1 |
Certification of Chief Executive Officer Pursuant to Section 302 of Sarbanes-Oxley Act of 2002 (15) |
| 31.2 |
Certification of Chief Financial Officer Pursuant to Section 302 of Sarbanes-Oxley Act of 2002 (15) |
| 32.1 |
Certification of Chief Executive Officer and Chief Financial Officer pursuant to Section 906 of the Sarbanes-Oxley Act of 2002 (15) |
| (1) |
Incorporated by reference to the Registrants Registration Statement on Form S-1 (No. 33-91094) declared effective on June 1, 1995. |
| (2) |
Incorporated by reference to the Registrants Registration Statement on Form 8-A12G filed on April 25, 1997. |
| (3) |
Incorporated by reference to the Registrants 1997 Proxy Statement. |
| (4) |
Incorporated by reference to the Registrants Report on Form 10-K dated June 30, 1998. |
| (5) |
Incorporated by reference to the Registrants Report on Form 10-K for the year ended June 30, 2001. |
| (6) |
Incorporated by reference to the Registrants Report on Form 10-K for the year ended June 30, 2002. |
| (7) |
Incorporated by reference to the Registrants 2003 Definitive Proxy Statement dated October 13, 2007. |
| (8) |
Incorporated by reference to the Registrants Form 8-K dated November 8, 2005. |
| (9) |
Incorporated by reference to the Registrants Form 8-K dated March 13, 2006. |
| (10) |
Incorporated by reference to the Registrants Form 8-K dated June 8, 2006. |
| (11) |
Incorporated by reference to the Registrants Report on Form 10-K for the year ended June 30, 2005. |
| (12) |
Incorporated by reference to the Registrants Report on Form 8-K dated February 23, 2007. |
| (13) |
Incorporated by reference to the Registrants Report on Form 10-Q for the quarter ended December 31, 2006. |
| (14) |
Incorporated by reference to the Registrants Report on Form 8-K dated July 9, 2007. |
| (15) |
Filed herewith. |
| (16) |
Incorporated by reference to the Registrants Report on Form 8-K dated November 9, 2006. |