AirFit F30, ResMed’s First Minimal-Contact Full Face CPAP Mask, Now Available in the U.S., Canada
Nearly 2 to 1 patients prefer AirFit F30 over the current minimal-contact full face market leader
SAN DIEGO--(BUSINESS WIRE)-- ResMed (NYSE: RMD, ASX: RMD) today announced the availability of its first minimal-contact full face CPAP mask, AirFit F30, the latest addition to its AirFit mask portfolio, which helps sleep apnea patients reduce facial marks, wear glasses in bed and curl up closer to their bed partner.
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20181015005185/en/
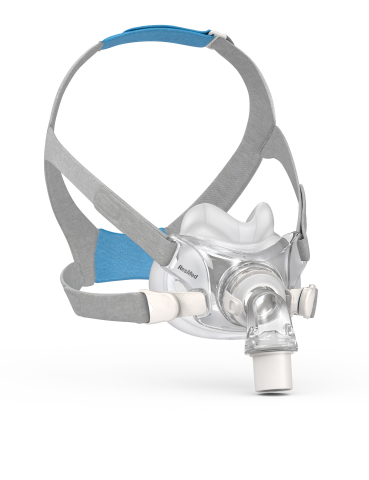
AirFit F30 full face CPAP mask: Side View (Photo: Business Wire)
Availability
AirFit F30 is available for sale now in the United States and Canada, and will be on display at the Medtrade Fall conference this week in Atlanta; visit ResMed’s Booth #2116.
AirFit F30 fits 93 percent of patients,1 and features a minimal-contact cushion that sits just below the nose, preventing top-of-the-nose red marks and irritation, plus reducing feelings of claustrophobia for some full face wearers. It also has ResMed’s QuietAir vent, making the mask quieter than ambient noise in the bedroom.
New claims: ResMed AirFit F30 vs. the competition
Nearly 2 to 1 patients preferred AirFit F30 over the current market leader in minimal-contact full face masks.2 When specifically asked about comfort, seal and ease of use, more patients picked AirFit F30 each time.2 AirFit F30 is also quieter than the current market leader, based on published performance (21 vs. 24 dBA),3 with fewer sleep disruptions reported due to airflow.2
“AirFit F30 is a clear choice over other minimal-contact full face masks,” said Jim Hollingshead, president of ResMed’s Sleep business. “We’re excited to now offer it, along with the rest of our full face, nasal and nasal pillows options, for an easy, comfortable, stable fit and a great night’s sleep.”
About ResMed
ResMed (NYSE: RMD, ASX: RMD), a world-leading connected health company with more than 6 million cloud-connected devices for daily remote patient monitoring, changes lives with every breath. Its award-winning devices and software solutions help treat and manage sleep apnea, chronic obstructive pulmonary disease and other respiratory conditions. Its 6,000-member team strives to improve patients’ quality of life, reduce the impact of chronic disease and save healthcare costs in more than 120 countries. ResMed.com
1 ResMed AirFit F30 fitting study of 75 patients conducted from April to
May 2018 in multiple U.S. locations.
2 ResMed AirFit F30 guided
clinical study of 23 patients conducted from June 8 to August 24 2018 in
Australia. Study tested ResMed AirFit F30 against Phillips Respironics
DreamWear full face (both masks are minimal-contact, full face).
3
ResMed AirFit user guide, 2018. Philips Respironics DreamWear full face
mask instructions for use, 2016.
View source version on businesswire.com: https://www.businesswire.com/news/home/20181015005185/en/
ResMed
For media:
Jayme Rubenstein
+1 858.836.6798
news@resmed.com
or
For
investors:
Amy Wakeham
+1 858.836.5000
investorrelations@resmed.com
Source: ResMed
Released October 15, 2018
